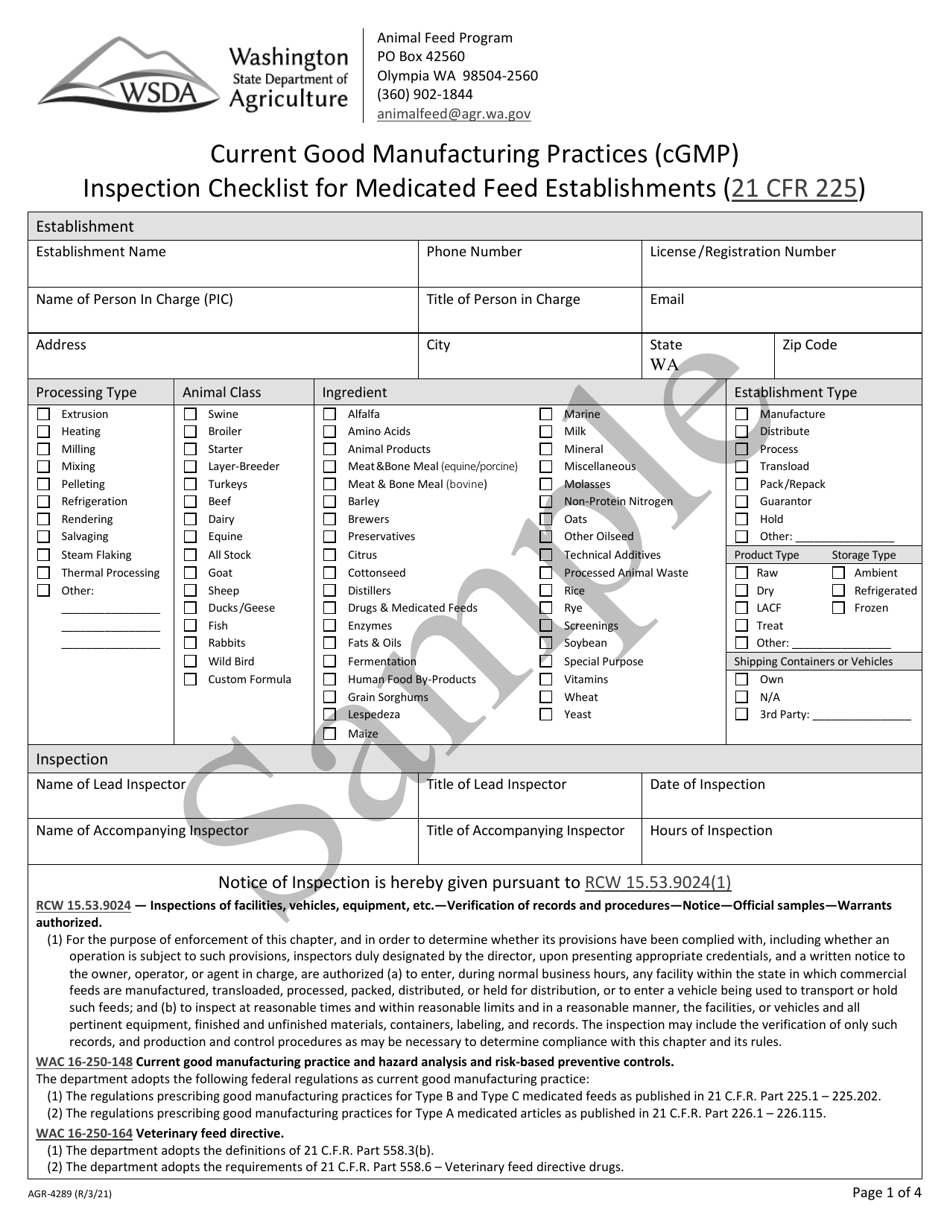
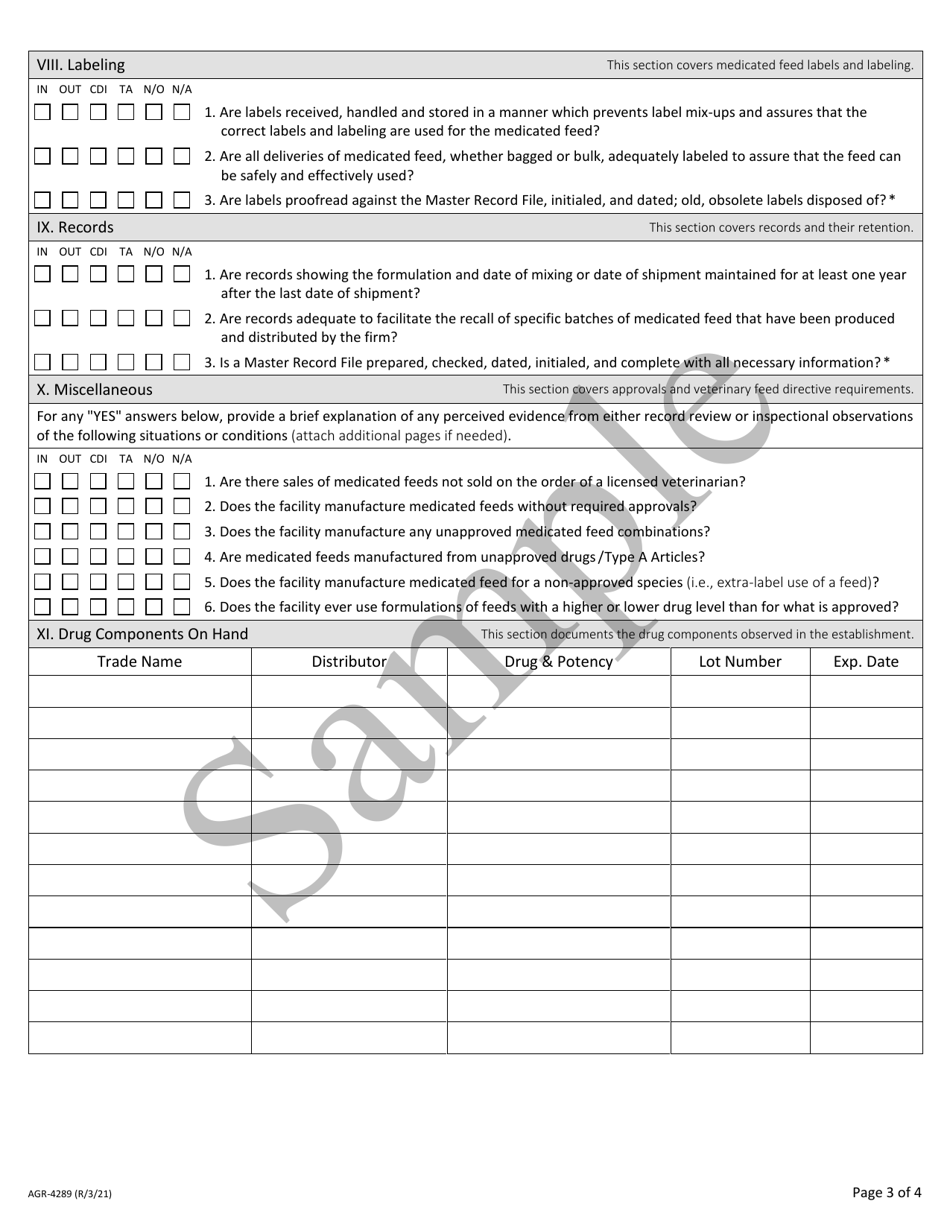
Form AGR-4289 Current Good Manufacturing Practices (Cgmp) Inspection Checklist for Medicated Feed Establishments (21 C.f.r. 225) - Sample - Washington
What Is Form AGR-4289?
This is a legal form that was released by the Washington State Department of Agriculture - a government authority operating within Washington. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is AGR-4289?
A: AGR-4289 is the Current Good Manufacturing Practices (CGMP) Inspection Checklist for Medicated Feed Establishments.
Q: What does CGMP stand for?
A: CGMP stands for Current Good Manufacturing Practices.
Q: What is the purpose of the CGMP Inspection Checklist?
A: The purpose of the CGMP Inspection Checklist is to ensure that medicated feed establishments comply with the requirements of 21 C.F.R. 225.
Q: What is 21 C.F.R. 225?
A: 21 C.F.R. 225 is the federal regulation that establishes the CGMP requirements for medicated feeds.
Q: What is a medicated feed establishment?
A: A medicated feed establishment is a facility that manufactures, distributes, or labels medicated animal feed.
Q: What is the Sample - Washington mentioned in the document title?
A: The Sample - Washington indicates that the document is a sample inspection checklist specific to the state of Washington.
Form Details:
- Released on March 1, 2021;
- The latest edition provided by the Washington State Department of Agriculture;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form AGR-4289 by clicking the link below or browse more documents and templates provided by the Washington State Department of Agriculture.