DOH Form 690-193 Drug Other Controlled Substance Registration Application - Washington
What Is DOH Form 690-193?
This is a legal form that was released by the Washington State Department of Health - a government authority operating within Washington. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is DOH Form 690-193?
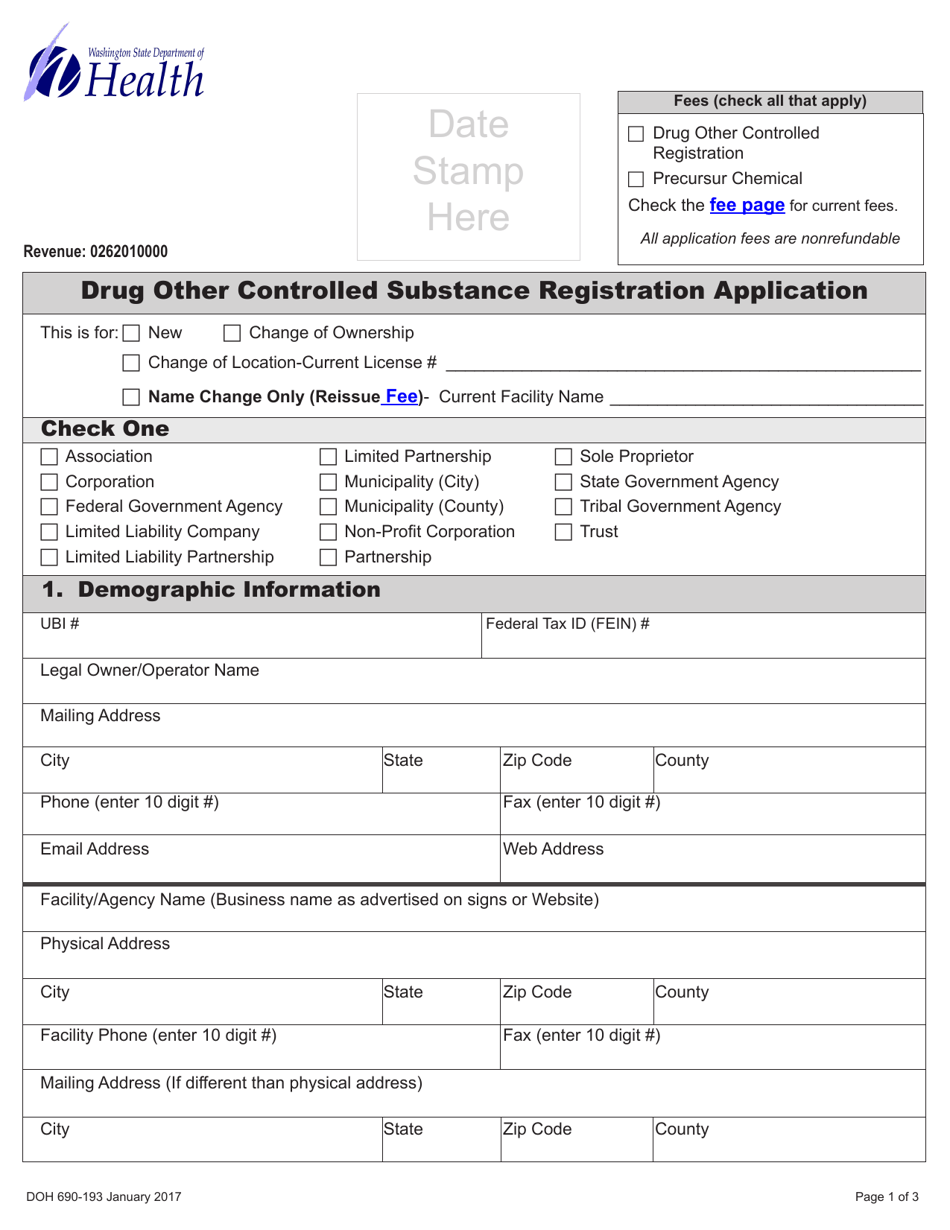
A: DOH Form 690-193 is the Drug Other Controlled Substance Registration Application form in Washington.
Q: What is the purpose of DOH Form 690-193?
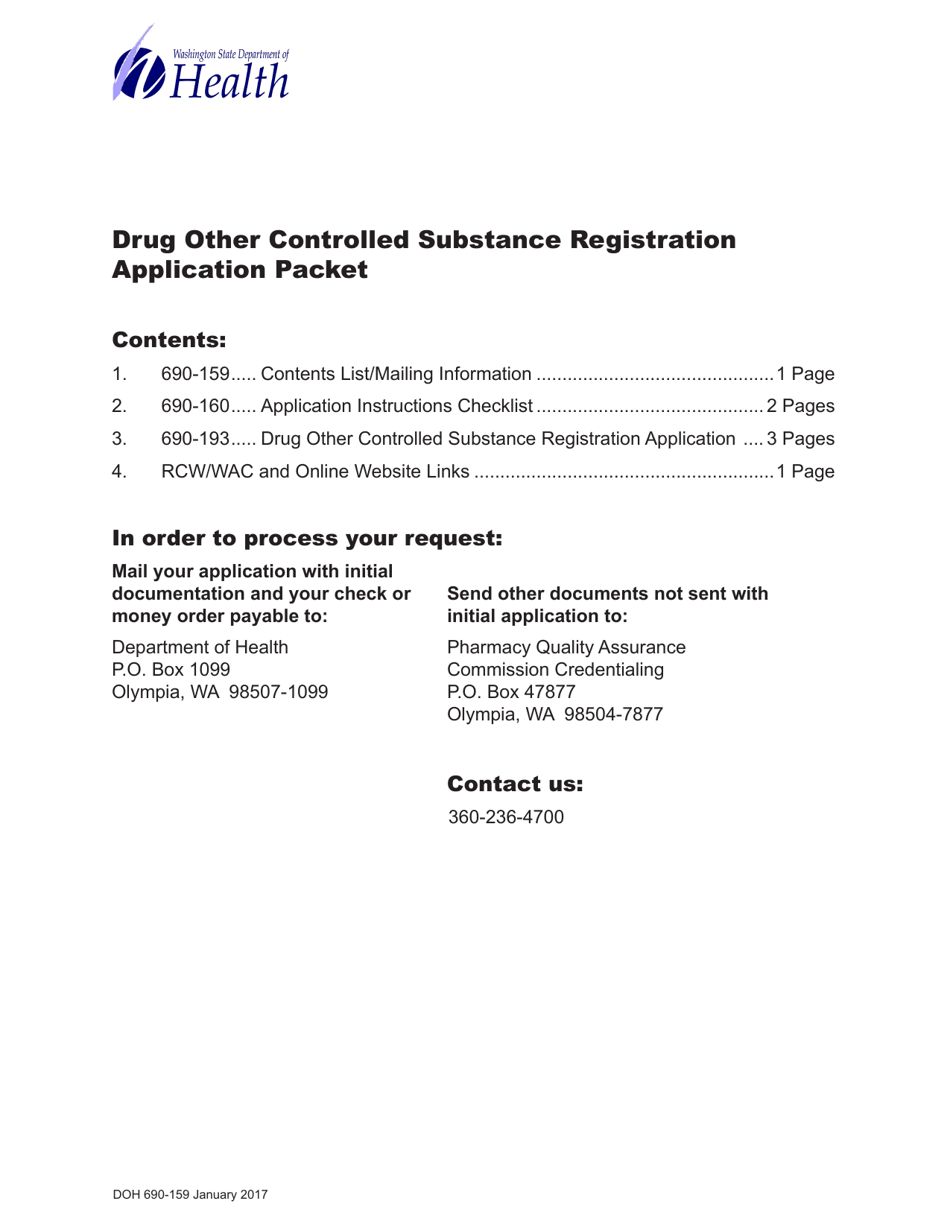
A: The purpose of DOH Form 690-193 is to apply for registration as a distributor, manufacturer, or dispenser of drugs and other controlled substances in Washington.
Q: Who needs to fill out DOH Form 690-193?
A: Anyone who wants to distribute, manufacture, or dispense drugs and other controlled substances in Washington needs to fill out DOH Form 690-193.
Q: Is there a fee for submitting DOH Form 690-193?
A: Yes, there is a fee for submitting DOH Form 690-193. The fee amount depends on the type of registration being applied for.
Q: Are there any prerequisites for filling out DOH Form 690-193?
A: Yes, there are prerequisites for filling out DOH Form 690-193. These include having a valid Washington state business license and an active DEA registration.
Q: How long does it take to process DOH Form 690-193?
A: The processing time for DOH Form 690-193 can vary. It is recommended to submit the application at least 90 days before the desired start date of registration.
Q: What documents do I need to submit along with DOH Form 690-193?
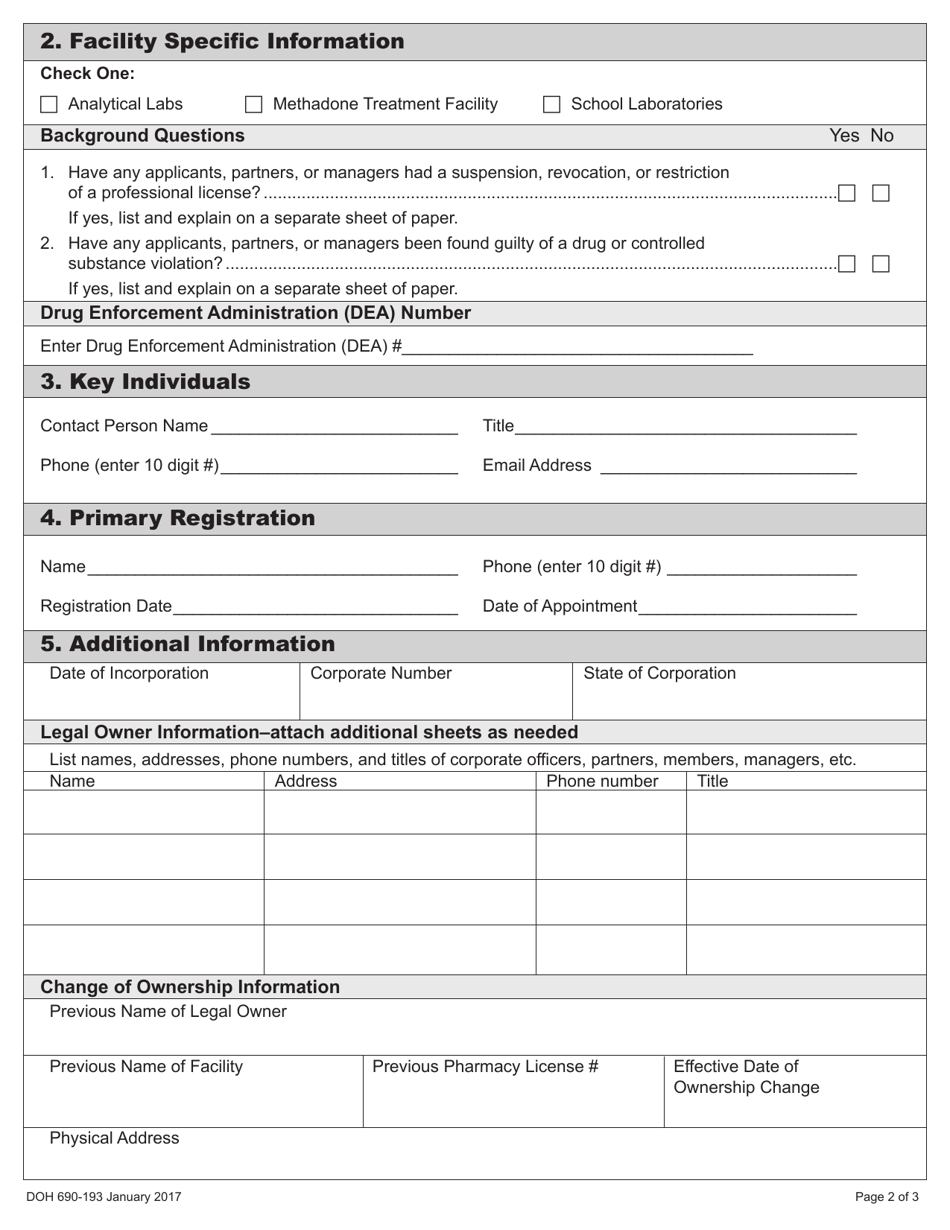
A: The required documents that need to be submitted with DOH Form 690-193 include a copy of the Washington state business license, a copy of the DEA registration, and additional supporting documentation.
Form Details:
- Released on January 1, 2017;
- The latest edition provided by the Washington State Department of Health;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of DOH Form 690-193 by clicking the link below or browse more documents and templates provided by the Washington State Department of Health.