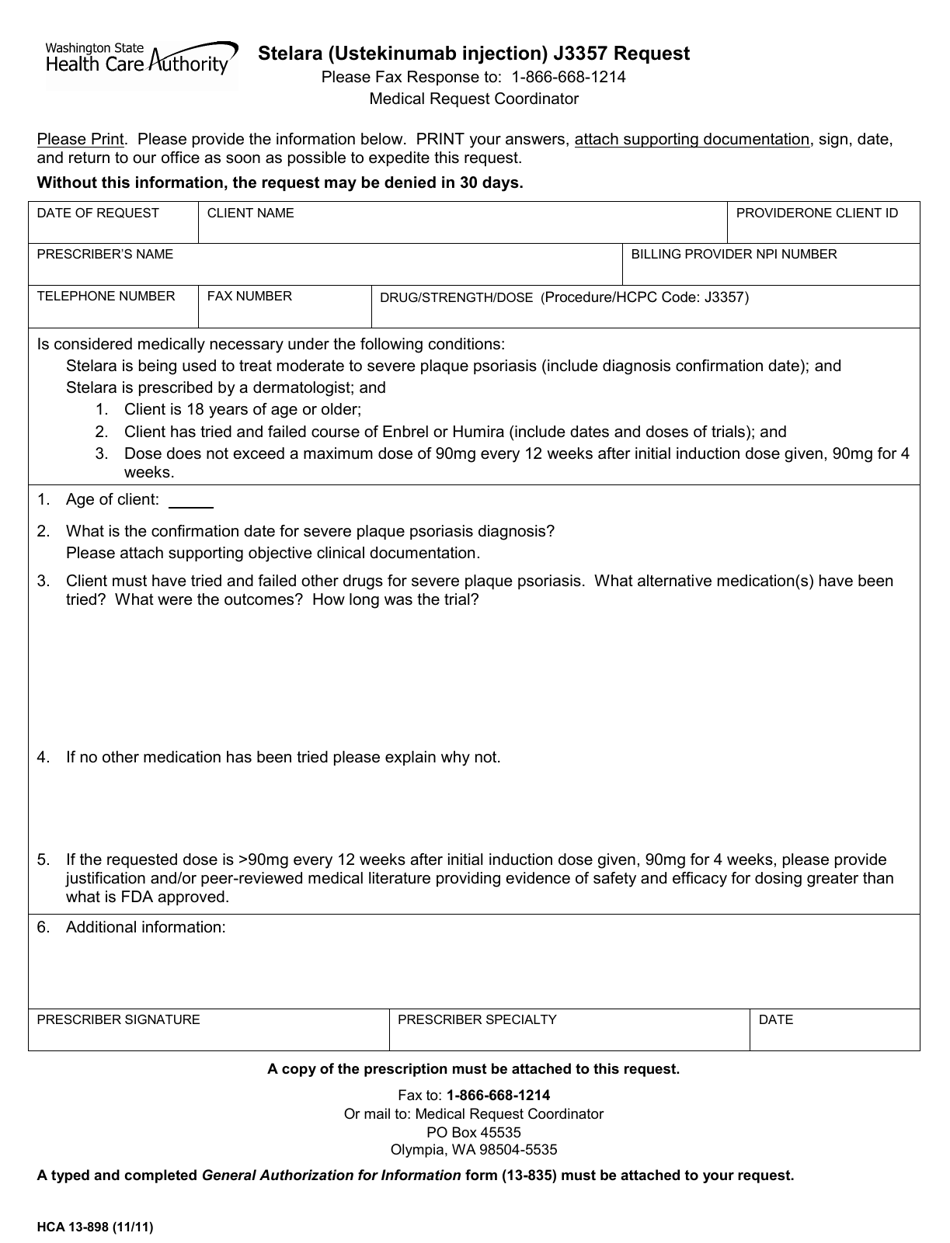
Form HCA13-898 Stelara (Ustekinumab Injection) J3357 Request - Washington
What Is Form HCA13-898?
This is a legal form that was released by the Washington State Health Care Authority - a government authority operating within Washington. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Stelara?
A: Stelara is a medication that contains the active ingredient Ustekinumab.
Q: What is Ustekinumab used for?
A: Ustekinumab is used to treat certain immune system disorders.
Q: What is Form HCA13-898?
A: Form HCA13-898 is a request form for Stelara (Ustekinumab Injection) J3357 in the state of Washington.
Q: What is the purpose of Form HCA13-898?
A: Form HCA13-898 is used to request authorization for the use of Stelara (Ustekinumab Injection) J3357.
Q: Is Stelara covered by insurance?
A: Coverage for Stelara may vary depending on individual insurance plans. It is best to check with your insurance provider.
Q: What are the possible side effects of Stelara?
A: Possible side effects of Stelara include infections, allergic reactions, and changes in liver function.
Q: Can Stelara be used during pregnancy?
A: The use of Stelara during pregnancy should be discussed with a healthcare provider, as it may pose potential risks.
Q: Is a prescription required for Stelara?
A: Yes, Stelara is a prescription medication and can only be obtained with a valid prescription from a healthcare provider.
Form Details:
- Released on November 1, 2011;
- The latest edition provided by the Washington State Health Care Authority;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of Form HCA13-898 by clicking the link below or browse more documents and templates provided by the Washington State Health Care Authority.