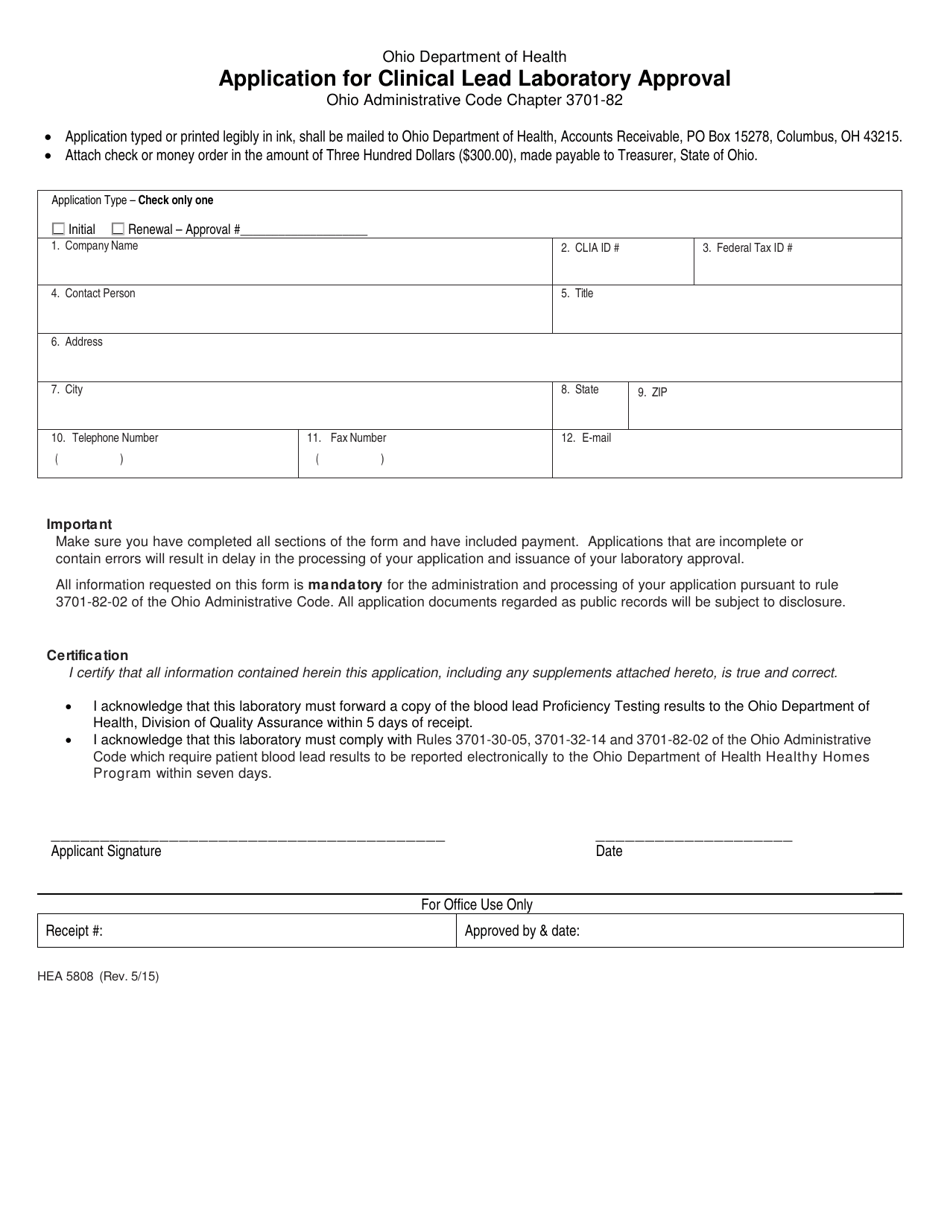
Form HEA5808 Application for Clinical Lead Laboratory Approval - Ohio
What Is Form HEA5808?
This is a legal form that was released by the Ohio Department of Health - a government authority operating within Ohio. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is HEA5808?
A: HEA5808 is the application form for Clinical Lead Laboratory Approval in Ohio.
Q: What is Clinical Lead Laboratory Approval?
A: Clinical Lead Laboratory Approval is the process by which a laboratory is certified to perform lead testing.
Q: Who needs to complete the HEA5808 application form?
A: Any laboratory seeking Clinical Lead Laboratory Approval in Ohio needs to complete the HEA5808 application form.
Q: Are there any fees associated with the HEA5808 application?
A: Yes, there is a fee associated with the HEA5808 application. Please see the application form for more details.
Q: What documents do I need to submit along with the HEA5808 application form?
A: You will need to submit various documents, including a completed application form, proof of accreditation, and quality control procedures.
Q: How long does it take to process the HEA5808 application?
A: The processing time for the HEA5808 application can vary. Please contact the Ohio Department of Health for more information.
Q: What happens after my HEA5808 application is approved?
A: Once your HEA5808 application is approved, your laboratory will be granted Clinical Lead Laboratory Approval and you will be able to perform lead testing.
Q: What if my HEA5808 application is denied?
A: If your HEA5808 application is denied, you will receive notification from the Ohio Department of Health with information on how to appeal the decision.
Q: Can I renew my Clinical Lead Laboratory Approval?
A: Yes, you can renew your Clinical Lead Laboratory Approval. Please see the Ohio Department of Health for more information on the renewal process.
Form Details:
- Released on May 1, 2015;
- The latest edition provided by the Ohio Department of Health;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form HEA5808 by clicking the link below or browse more documents and templates provided by the Ohio Department of Health.