Administrative Changes - Certification Form for Veterinary Drugs - Canada
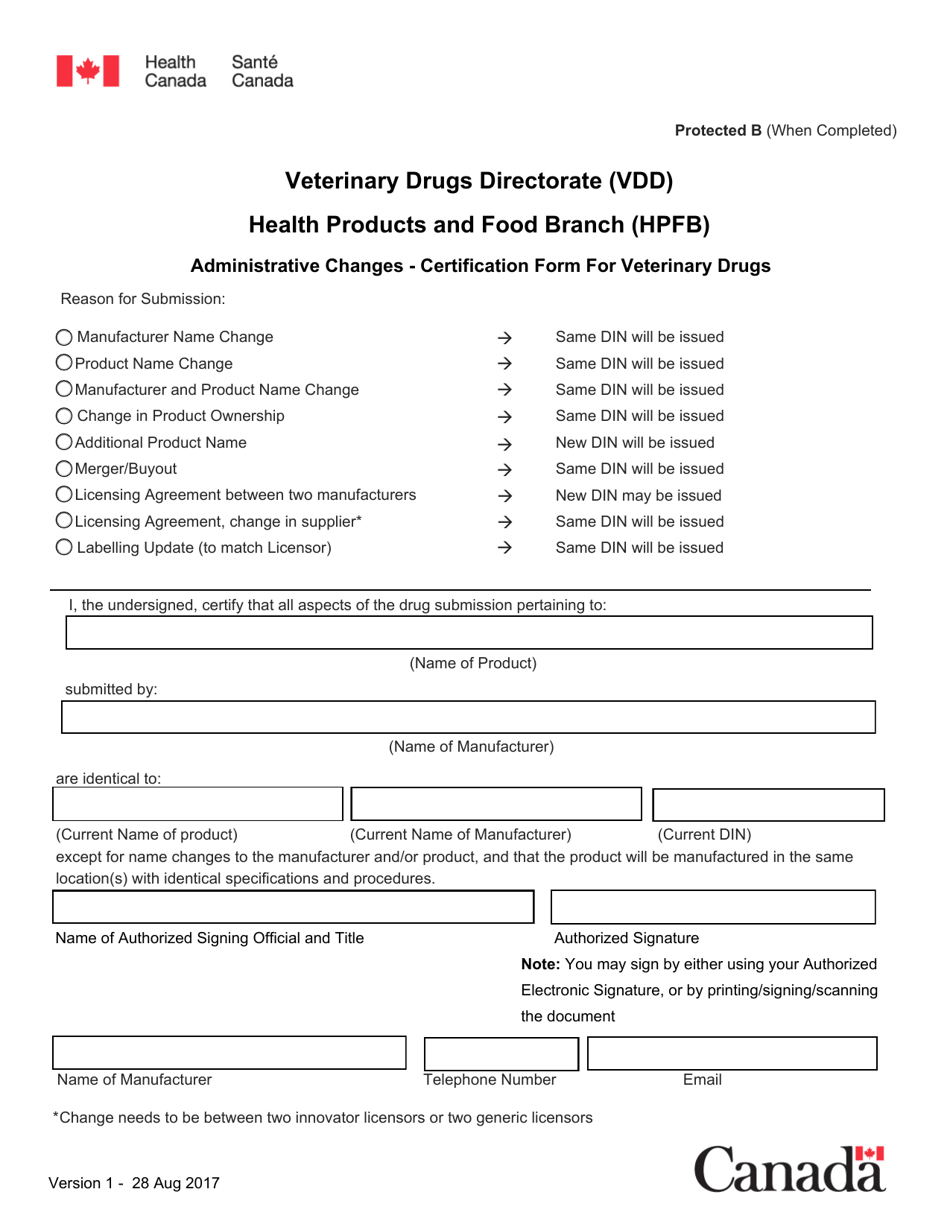
The Administrative Changes - Certification Form for Veterinary Drugs in Canada is used for making administrative changes to the packaging, labeling, or product information of veterinary drugs that do not impact the product's safety or effectiveness. It allows for the certification and submission of these changes to Health Canada's Veterinary Drugs Directorate.
In Canada, the Health Products and Food Branch (HPFB) of Health Canada processes and reviews the Administrative Changes - Certification Form for Veterinary Drugs.
FAQ
Q: What are the administrative changes for the certification form for veterinary drugs in Canada?
A: The administrative changes for the certification form for veterinary drugs in Canada involve updates to the form itself and the process for submitting it.
Q: What is the purpose of the certification form for veterinary drugs in Canada?
A: The certification form for veterinary drugs in Canada is used to ensure the quality and safety of veterinary drugs being imported into the country.
Q: How do I submit the certification form for veterinary drugs in Canada?
A: The process for submitting the certification form for veterinary drugs in Canada may vary depending on the specific requirements set by the regulatory authority. It is recommended to consult the official guidelines or contact the relevant authority for instructions.
Q: What are the consequences of not submitting the certification form for veterinary drugs in Canada?
A: Failing to submit the certification form for veterinary drugs in Canada or not meeting the specified requirements may result in the rejection of the import application or other legal consequences. It is important to comply with the regulations to avoid any issues.