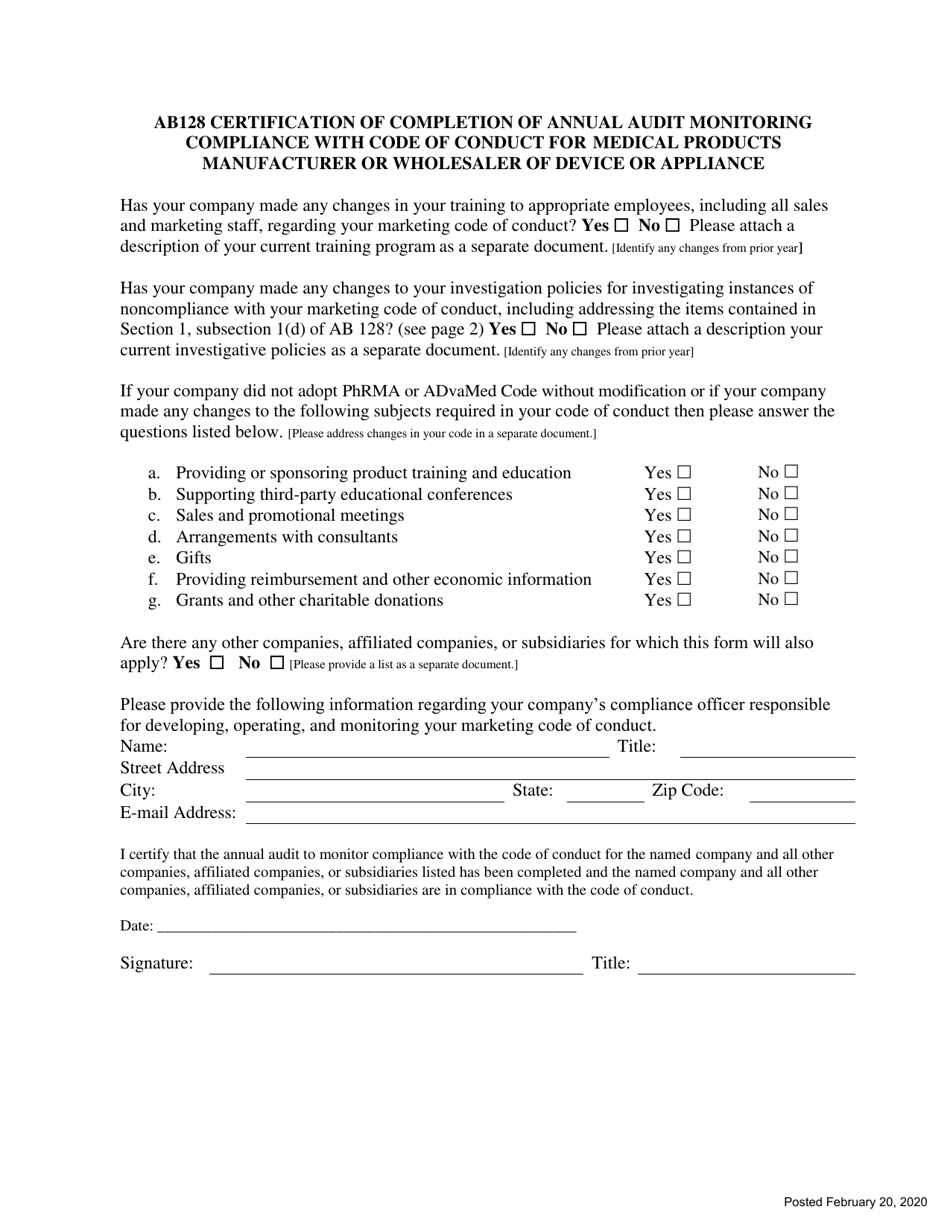
Ab128 Certification of Completion of Annual Audit Monitoring Compliance With Code of Conduct for Manufacturers and Wholesalers of Drugs, Medicines, Chemicals, Devices, or Appliances - Nevada
Ab128 Certification of Completion of Annual Audit Monitoring Compliance With Code of Conduct for Manufacturers and Wholesalers of Drugs, Medicines, Chemicals, Devices, or Appliances is a legal document that was released by the Nevada Department of Health and Human Services - a government authority operating within Nevada.
FAQ
Q: What is the AB128 Certification?
A: The AB128 Certification is a document that certifies the completion of an annual audit monitoring compliance with the Code of Conduct for Manufacturers and Wholesalers of Drugs, Medicines, Chemicals, Devices, or Appliances in Nevada.
Q: Who needs to have the AB128 Certification?
A: Manufacturers and wholesalers of drugs, medicines, chemicals, devices, or appliances in Nevada need to have the AB128 Certification.
Q: What does the AB128 Certification monitor?
A: The AB128 Certification monitors compliance with the Code of Conduct for Manufacturers and Wholesalers of Drugs, Medicines, Chemicals, Devices, or Appliances.
Q: Why is the AB128 Certification important?
A: The AB128 Certification is important to ensure that manufacturers and wholesalers of drugs, medicines, chemicals, devices, or appliances in Nevada are following the established code of conduct.
Q: How often is the AB128 Certification required?
A: The AB128 Certification is required to be completed annually.
Form Details:
- Released on February 20, 2020;
- The latest edition currently provided by the Nevada Department of Health and Human Services;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of the form by clicking the link below or browse more documents and templates provided by the Nevada Department of Health and Human Services.