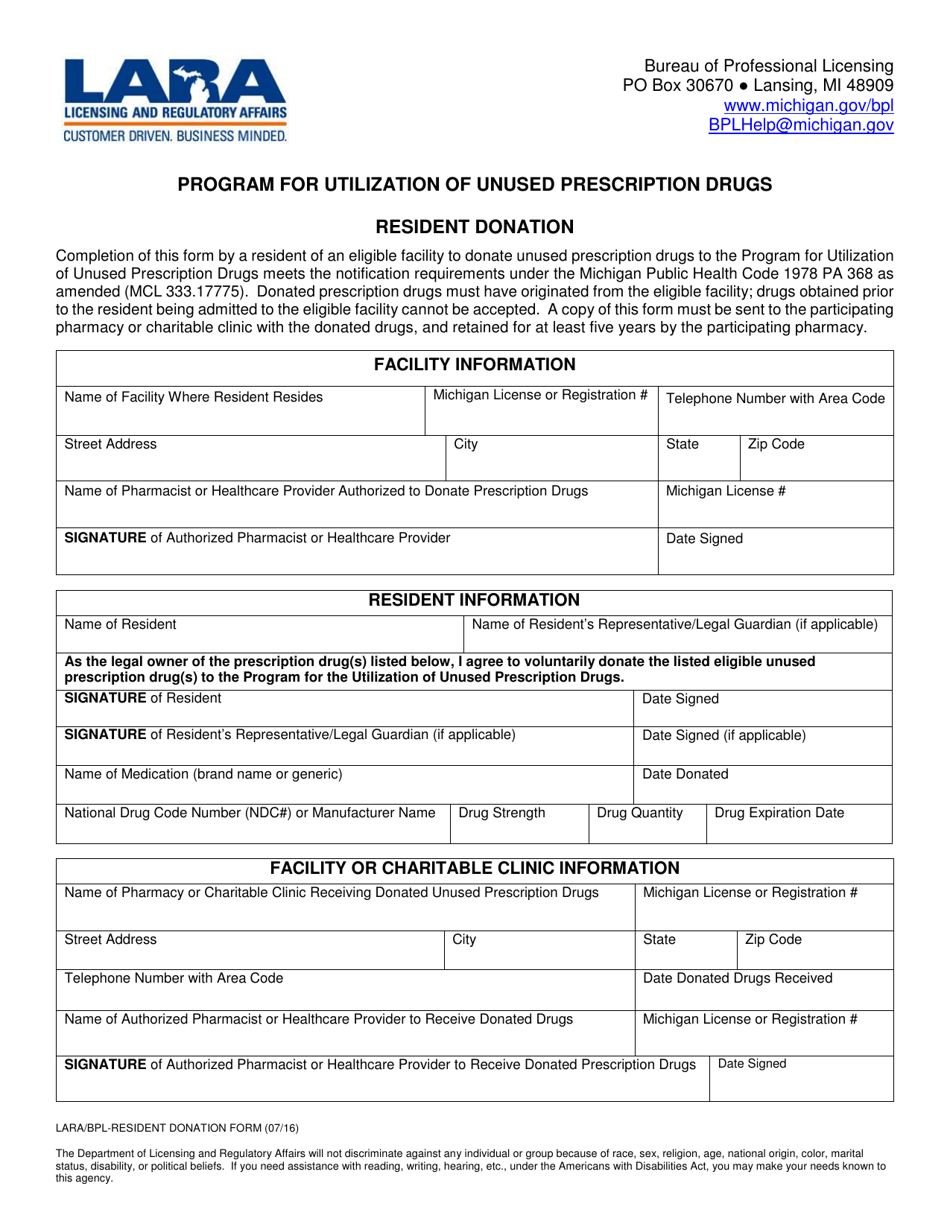
Resident Donation - Program for Utilization of Unused Prescription Drugs - Michigan
Resident Donation - Program for Utilization of Unused Prescription Drugs is a legal document that was released by the Michigan Department of Licensing and Regulatory Affairs - a government authority operating within Michigan.
FAQ
Q: What is the Resident Donation Program for Utilization of Unused Prescription Drugs?
A: The Resident Donation Program for Utilization of Unused Prescription Drugs is a program in Michigan that allows residents to donate their unused prescription drugs.
Q: Why was the program established?
A: The program was established to help individuals who may not have access to affordable medications.
Q: Who can participate in the program?
A: Any resident of Michigan can participate in the program by donating their unused prescription drugs.
Q: What types of medications can be donated?
A: Most prescription medications can be donated, as long as they are in their original packaging and have not expired.
Q: How can someone donate their prescription drugs?
A: Residents can donate their prescription drugs by contacting a participating pharmacy in Michigan.
Q: Are there any restrictions on donating prescription drugs?
A: Yes, controlled substances and medications with special storage requirements cannot be donated.
Q: What happens to the donated prescription drugs?
A: The donated prescription drugs are inspected and sorted by participating pharmacies and then made available to eligible individuals in need.
Q: Is there any cost to participate in the program?
A: No, there is no cost for residents to participate in the Resident Donation Program for Utilization of Unused Prescription Drugs.
Q: Can individuals receive donated medications through the program?
A: Yes, eligible individuals in need can receive donated medications through the program.
Q: Is the program available in other states?
A: The program is currently only available in Michigan.
Form Details:
- Released on July 1, 2016;
- The latest edition currently provided by the Michigan Department of Licensing and Regulatory Affairs;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of the form by clicking the link below or browse more documents and templates provided by the Michigan Department of Licensing and Regulatory Affairs.