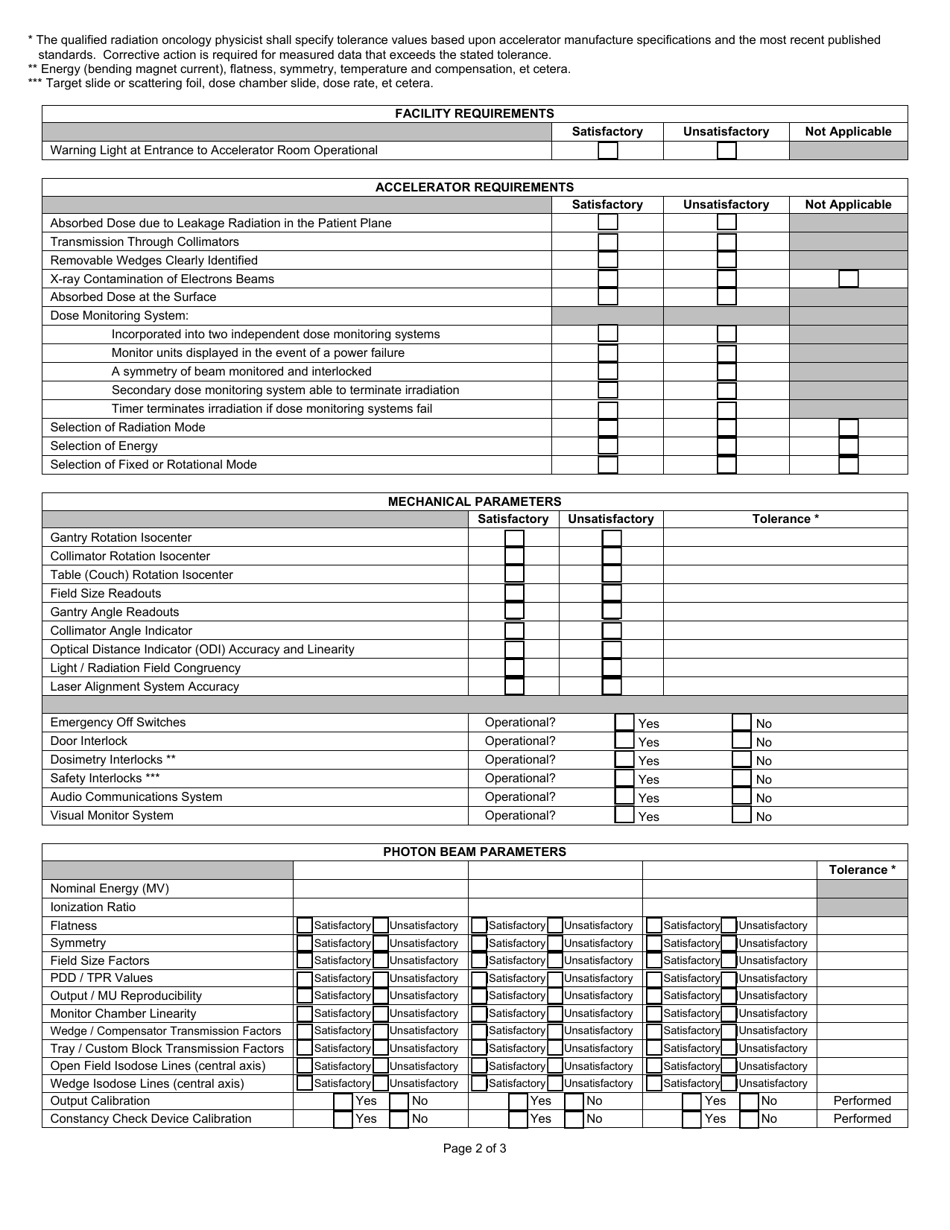
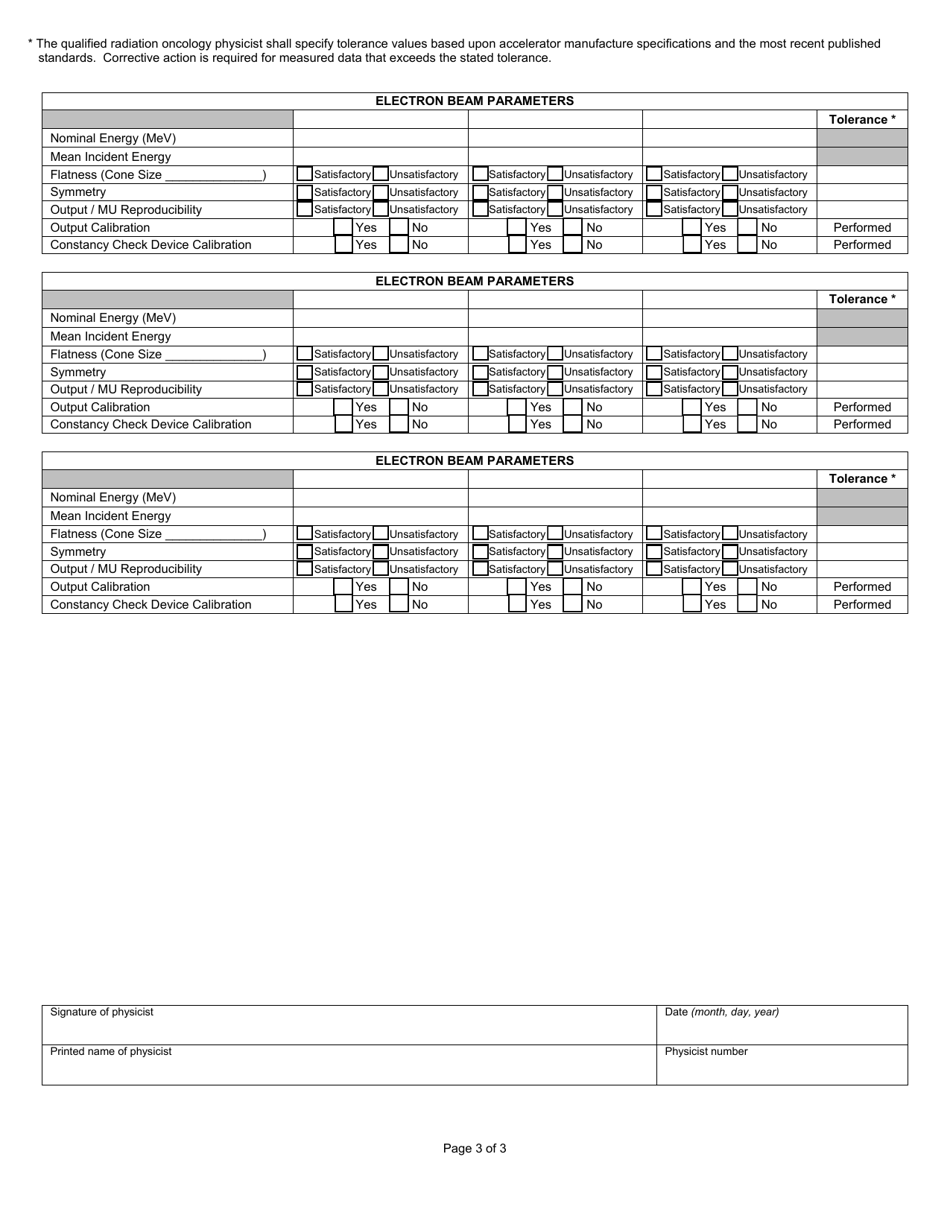
State Form 46886 Therapy Accelerator Initial Commissioning Summary - Indiana
What Is State Form 46886?
This is a legal form that was released by the Indiana State Department of Health - a government authority operating within Indiana. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is State Form 46886?
A: State Form 46886 is a document related to the Therapy Accelerator Initial Commissioning Summary in Indiana.
Q: What is the Therapy Accelerator Initial Commissioning Summary?
A: The Therapy Accelerator Initial Commissioning Summary is a report that provides a summary of the commissioning process for a therapy accelerator in Indiana.
Q: What is a therapy accelerator?
A: A therapy accelerator is a device used in the field of healthcare to assist with various therapies and treatments.
Q: What is commissioning?
A: Commissioning is the process of ensuring that a device or system is installed, tested, and functioning correctly according to its intended design and specifications.
Q: Who is required to complete the Therapy Accelerator Initial Commissioning Summary?
A: The completion of the Therapy Accelerator Initial Commissioning Summary is required for individuals or organizations that have installed a therapy accelerator in Indiana.
Q: What information is included in the Therapy Accelerator Initial Commissioning Summary?
A: The Therapy Accelerator Initial Commissioning Summary includes details about the device, installation process, testing procedures, and any necessary corrective actions.
Q: What is the purpose of the Therapy Accelerator Initial Commissioning Summary?
A: The purpose of the Therapy Accelerator Initial Commissioning Summary is to verify that a therapy accelerator has been properly commissioned and is ready for use in Indiana.
Q: Are there any fees associated with submitting the Therapy Accelerator Initial Commissioning Summary?
A: Fees may be required for the submission of the Therapy Accelerator Initial Commissioning Summary. It is advisable to check with the relevant regulatory authority for the current fee schedule.
Q: Is the Therapy Accelerator Initial Commissioning Summary applicable only to Indiana?
A: Yes, the Therapy Accelerator Initial Commissioning Summary is specific to Indiana and its regulations regarding therapy accelerator devices.
Form Details:
- Released on May 1, 2012;
- The latest edition provided by the Indiana State Department of Health;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of State Form 46886 by clicking the link below or browse more documents and templates provided by the Indiana State Department of Health.