This version of the form is not currently in use and is provided for reference only. Download this version of
Form FRM-0033
for the current year.
Form FRM-0033 Drug Establishment Licence (Del) Application Form - Canada
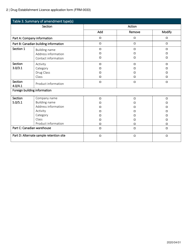
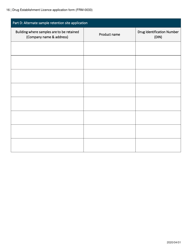
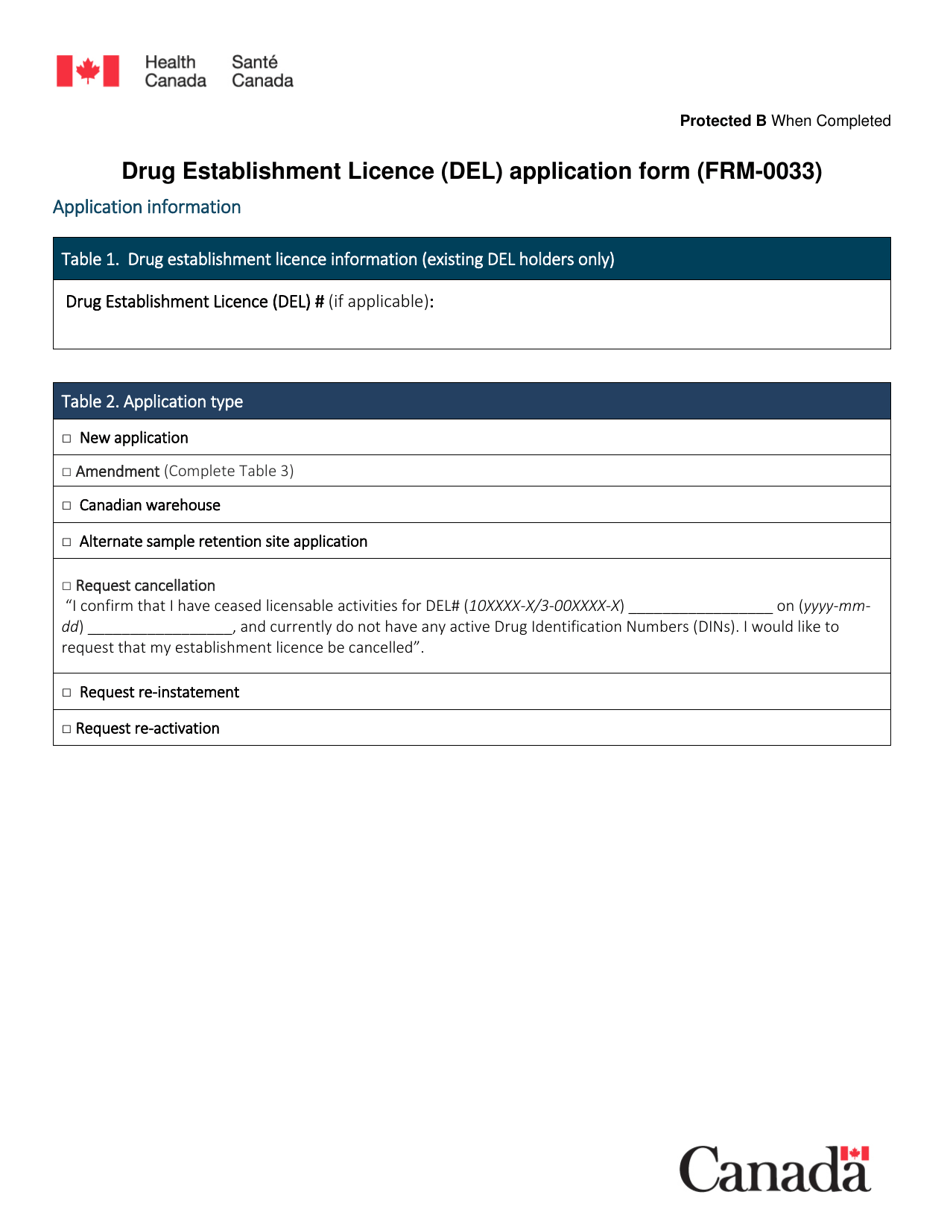
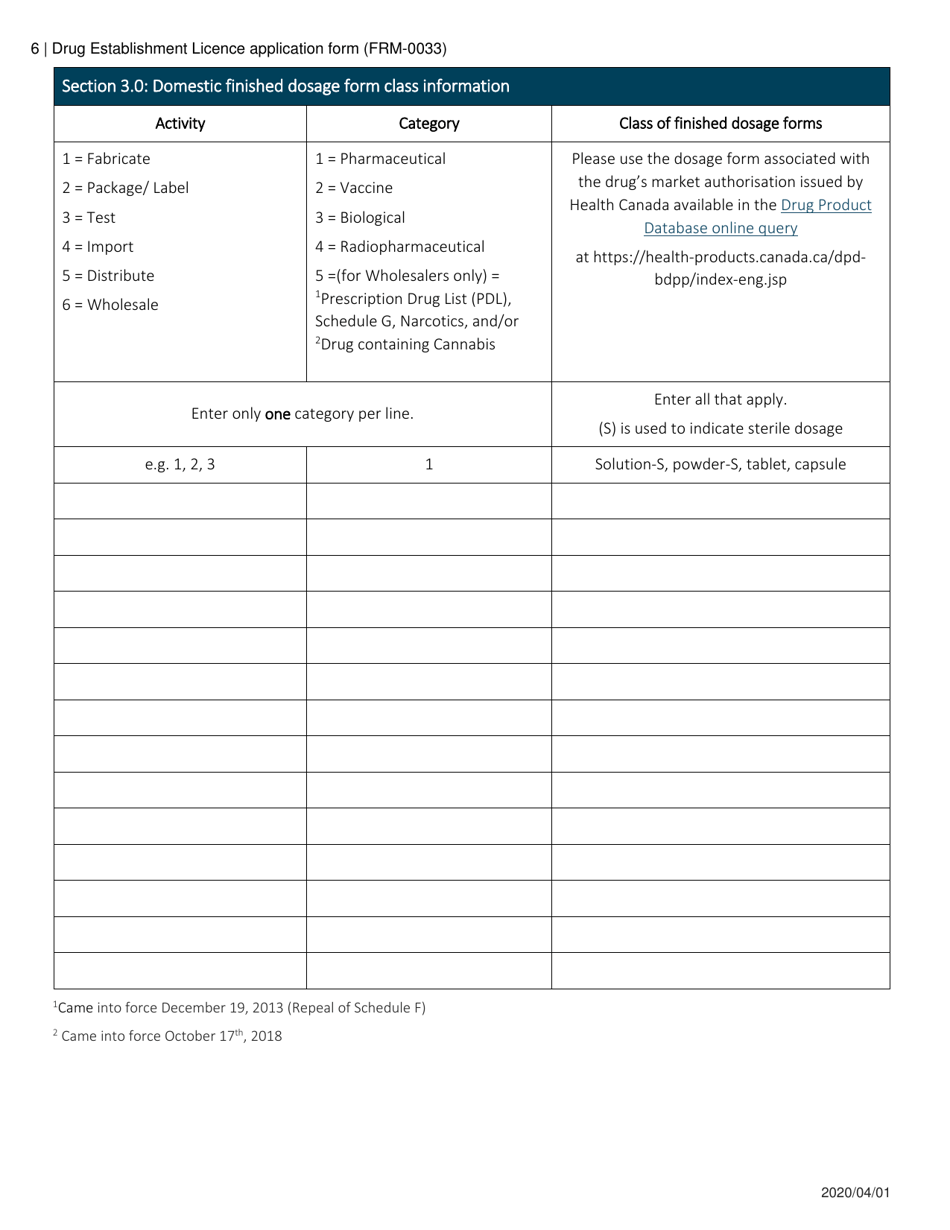
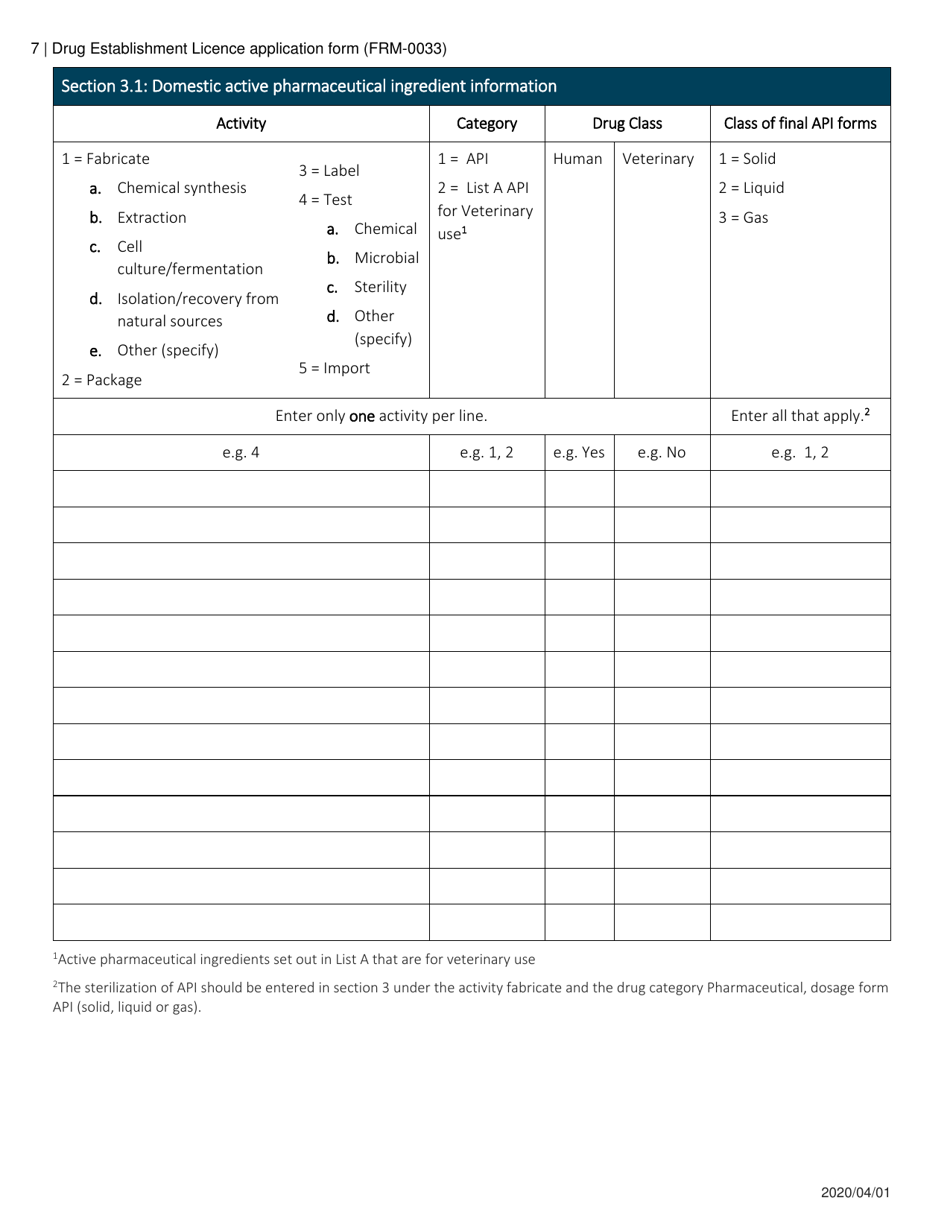
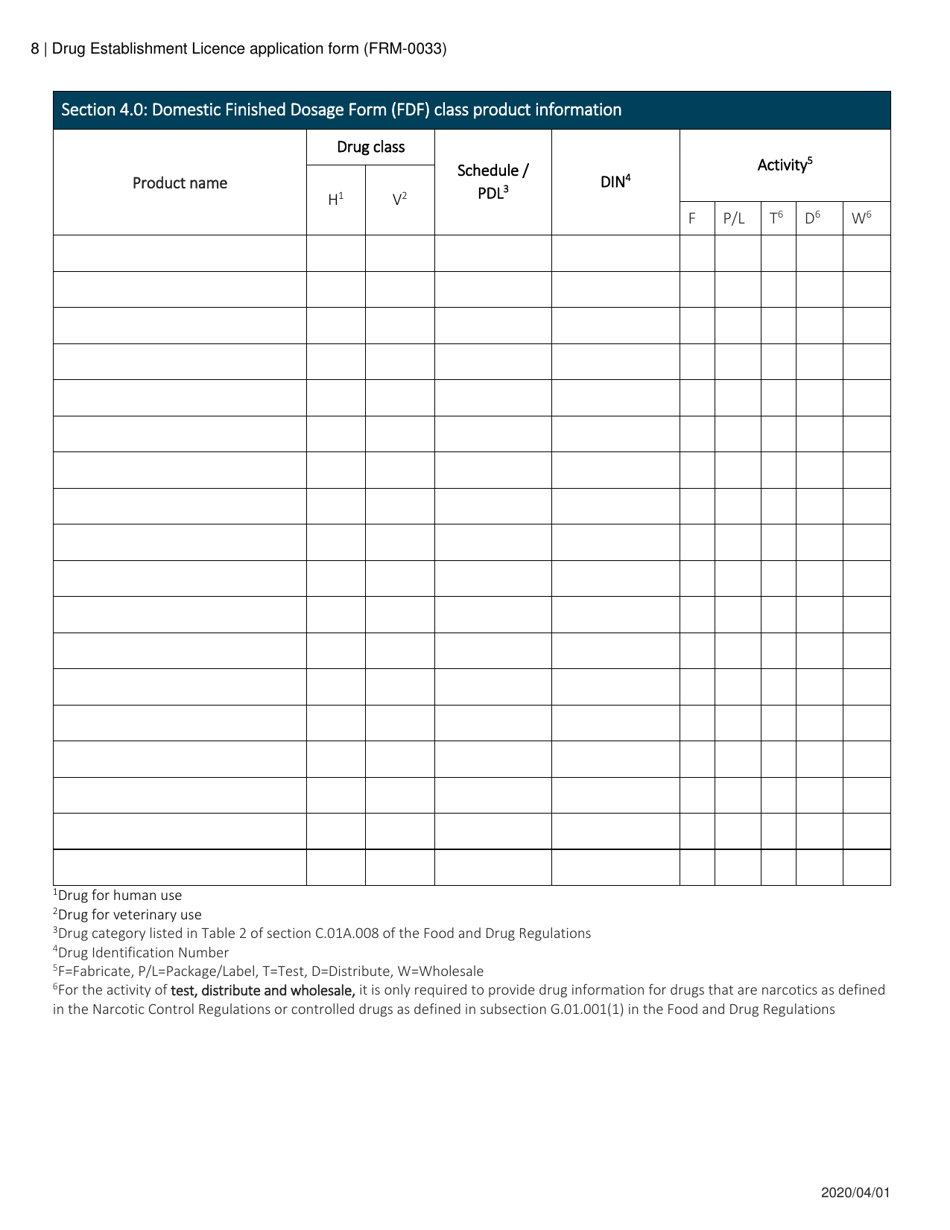
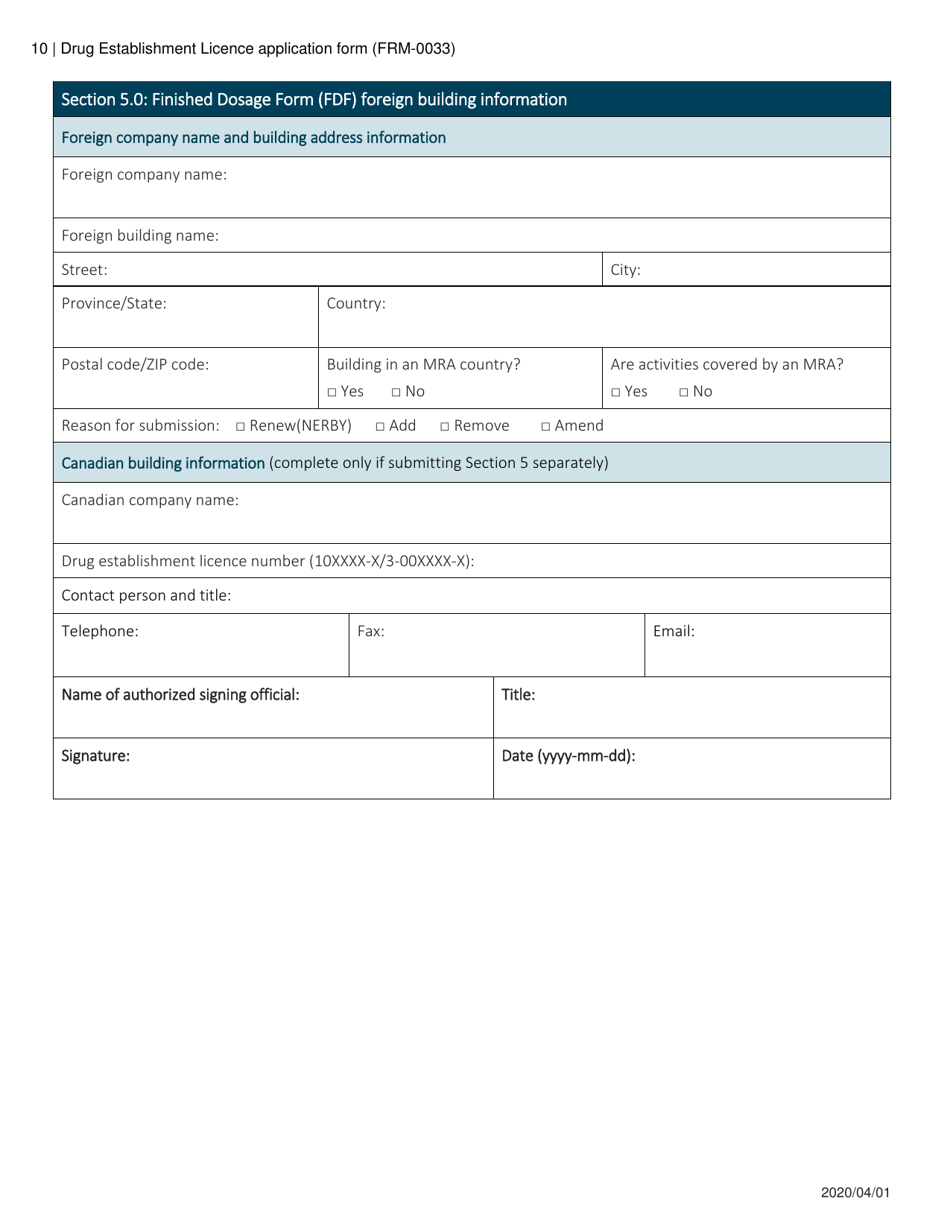
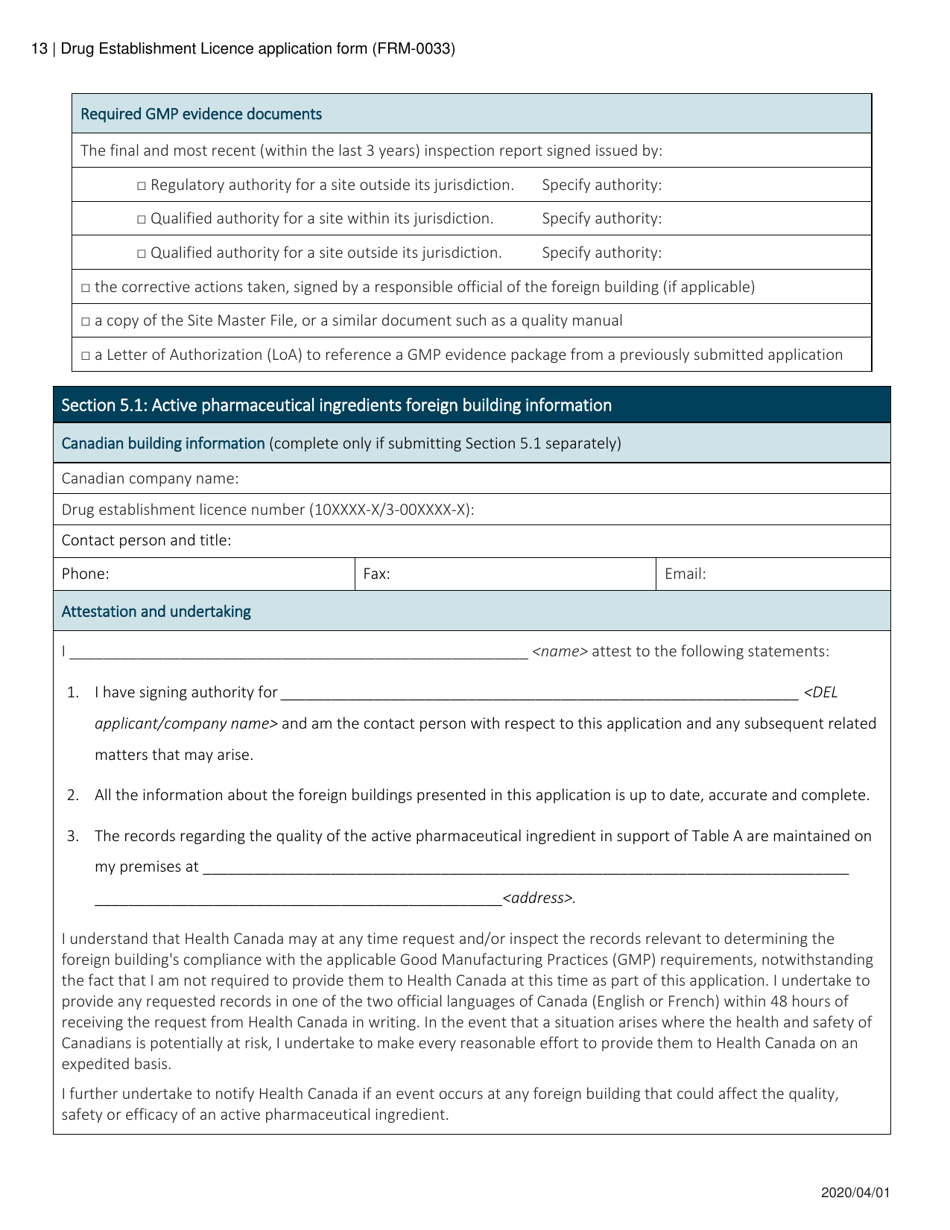
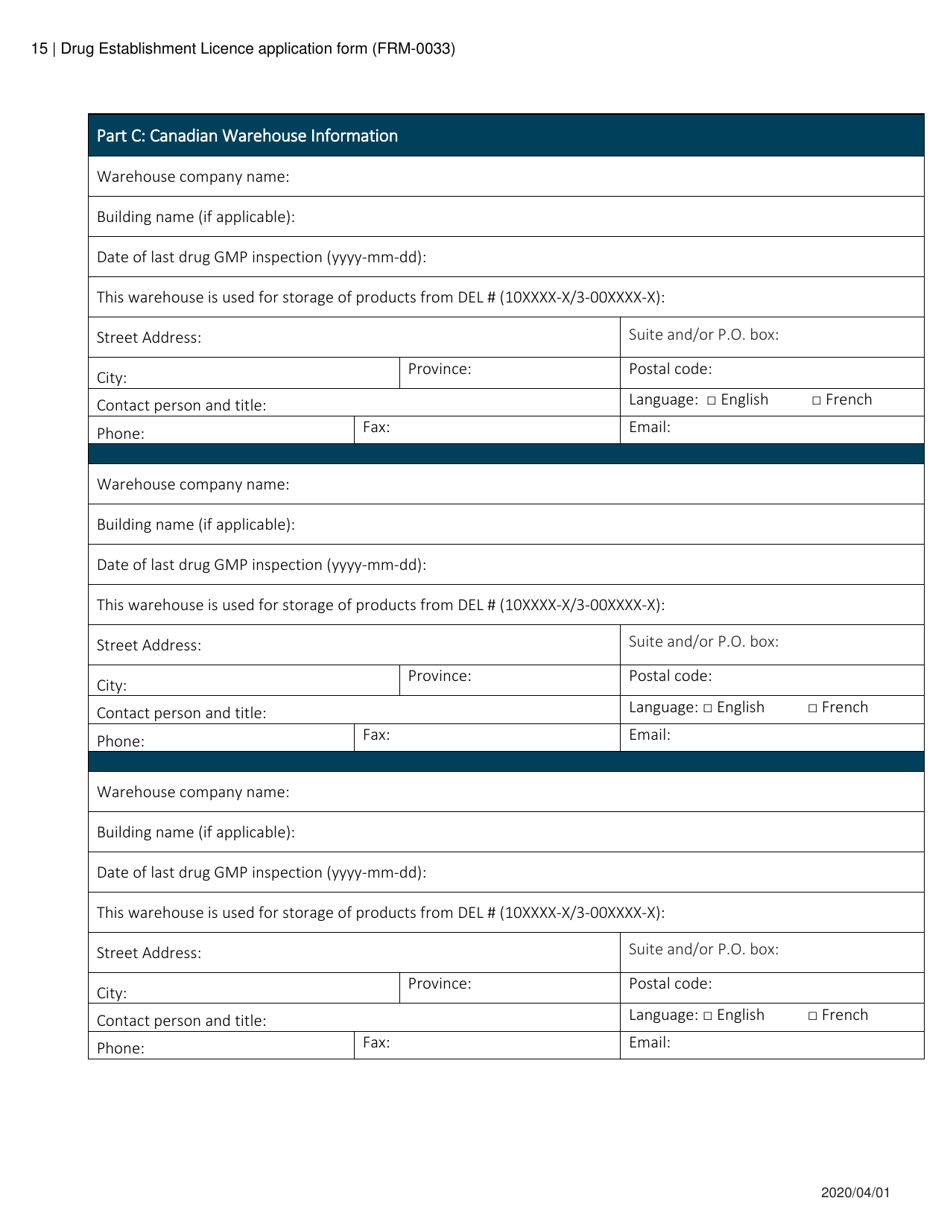
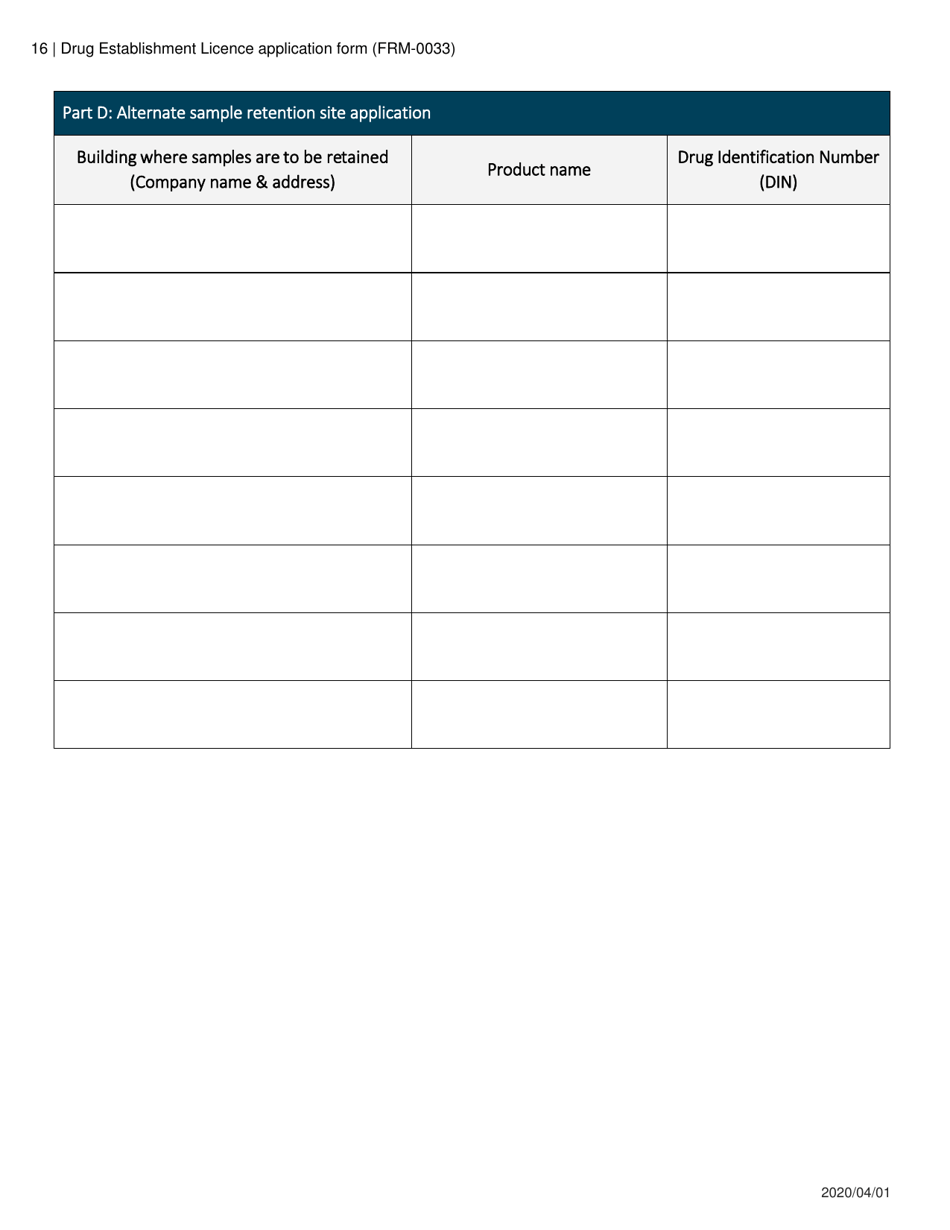
Form FRM-0033 Drug Establishment Licence (Del) Application Form in Canada is used for the application of a Drug Establishment Licence (DEL). A DEL is required for entities involved in the manufacturing, packaging, labeling, and testing of drugs in Canada. This form helps individuals and organizations apply for the necessary license to operate a drug establishment in Canada.
The Form FRM-0033 Drug Establishment Licence (DEL) Application Form in Canada is filed by the drug establishment that wishes to obtain a license for their operations.
FAQ
Q: What is the purpose of Form FRM-0033?
A: Form FRM-0033 is a Drug Establishment Licence (DEL) application form in Canada.
Q: Who needs to fill out Form FRM-0033?
A: Any organization or individual who wants to apply for a Drug Establishment Licence (DEL) in Canada needs to fill out Form FRM-0033.
Q: What information is required in Form FRM-0033?
A: Form FRM-0033 requires information such as the name and address of the establishment, the types of drugs to be manufactured, imported, or distributed, and the name and contact information of the designated representative.