This version of the form is not currently in use and is provided for reference only. Download this version of
the document
for the current year.
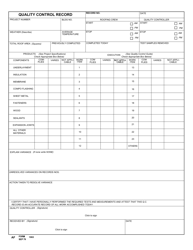
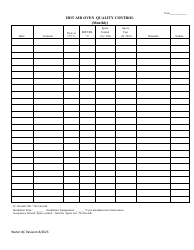
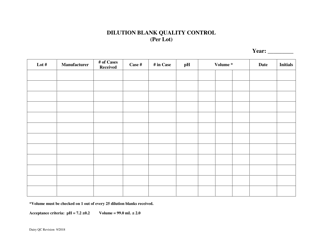
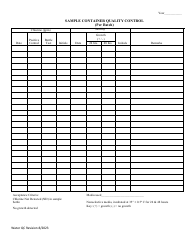
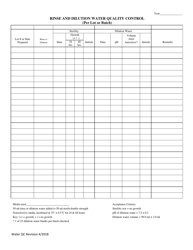
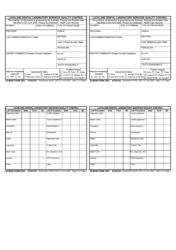
Autoclave Quality Control - Monthly / Quarterly - Illinois
Autoclave Quality Control - Monthly/Quarterly is a legal document that was released by the Illinois Department of Public Health - a government authority operating within Illinois.
FAQ
Q: What is autoclave quality control?
A: Autoclave quality control is a process that ensures the proper functioning and effectiveness of autoclaves, which are devices used for sterilization in various industries, including healthcare and laboratories.
Q: How often should autoclave quality control be done?
A: Autoclave quality control should be done on a regular basis, typically on a monthly or quarterly basis.
Q: What is the purpose of monthly/quarterly autoclave quality control in Illinois?
A: The purpose of monthly/quarterly autoclave quality control in Illinois is to ensure that autoclaves in the state are functioning properly and producing sterile results, in compliance with industry standards and regulations.
Q: Who should perform autoclave quality control?
A: Autoclave quality control should be performed by trained professionals, such as technicians or engineers, who have knowledge and experience in operating and maintaining autoclaves.
Q: What are some of the tests performed during autoclave quality control?
A: Some of the tests performed during autoclave quality control include temperature and pressure calibration, sterilization effectiveness testing, and overall equipment performance evaluation.
Form Details:
- Released on March 1, 2015;
- The latest edition currently provided by the Illinois Department of Public Health;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of the form by clicking the link below or browse more documents and templates provided by the Illinois Department of Public Health.