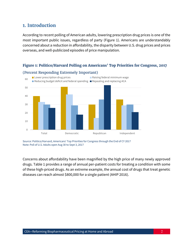
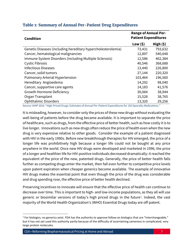
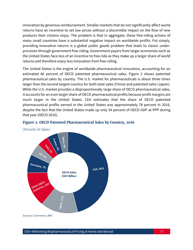
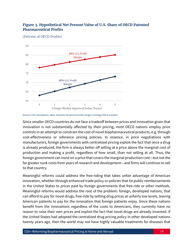
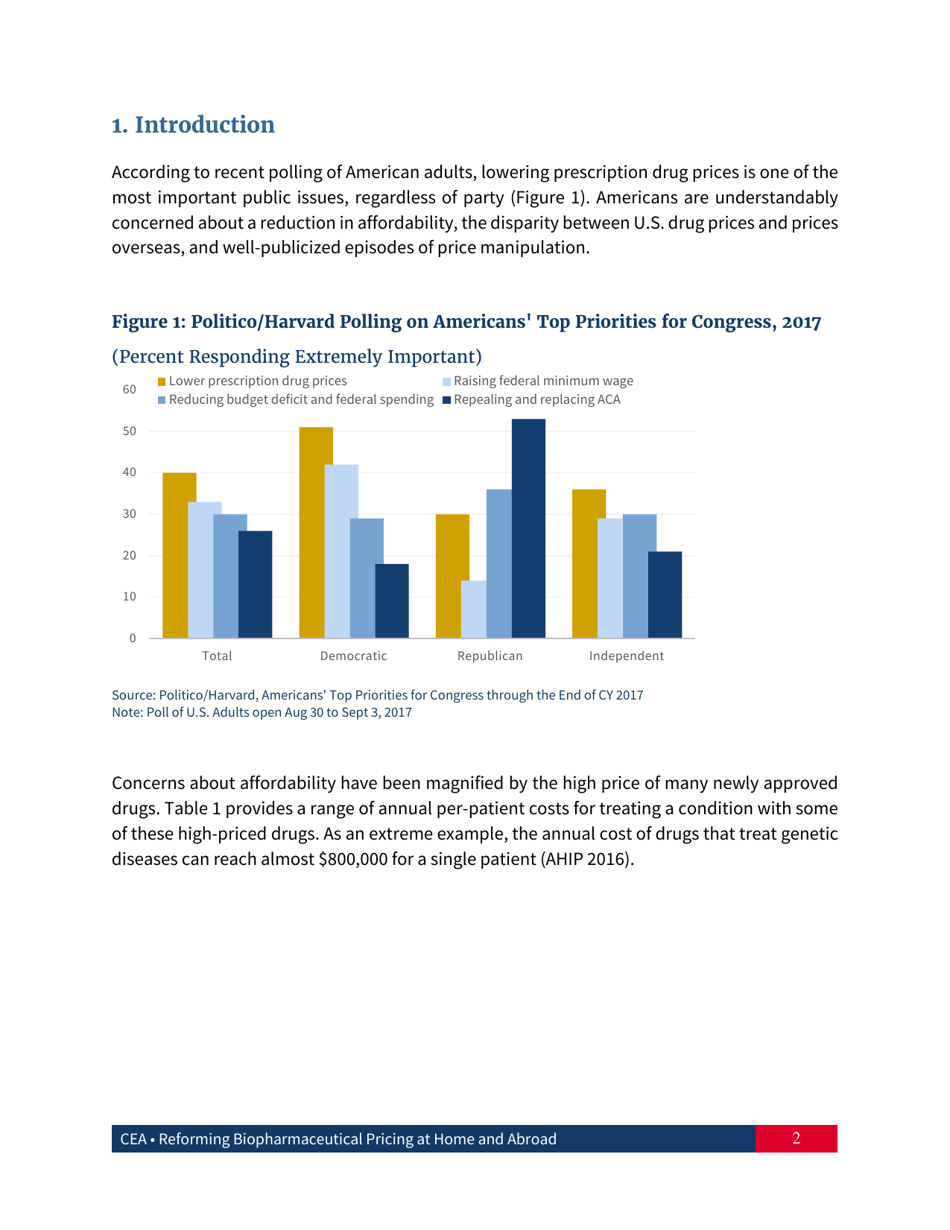
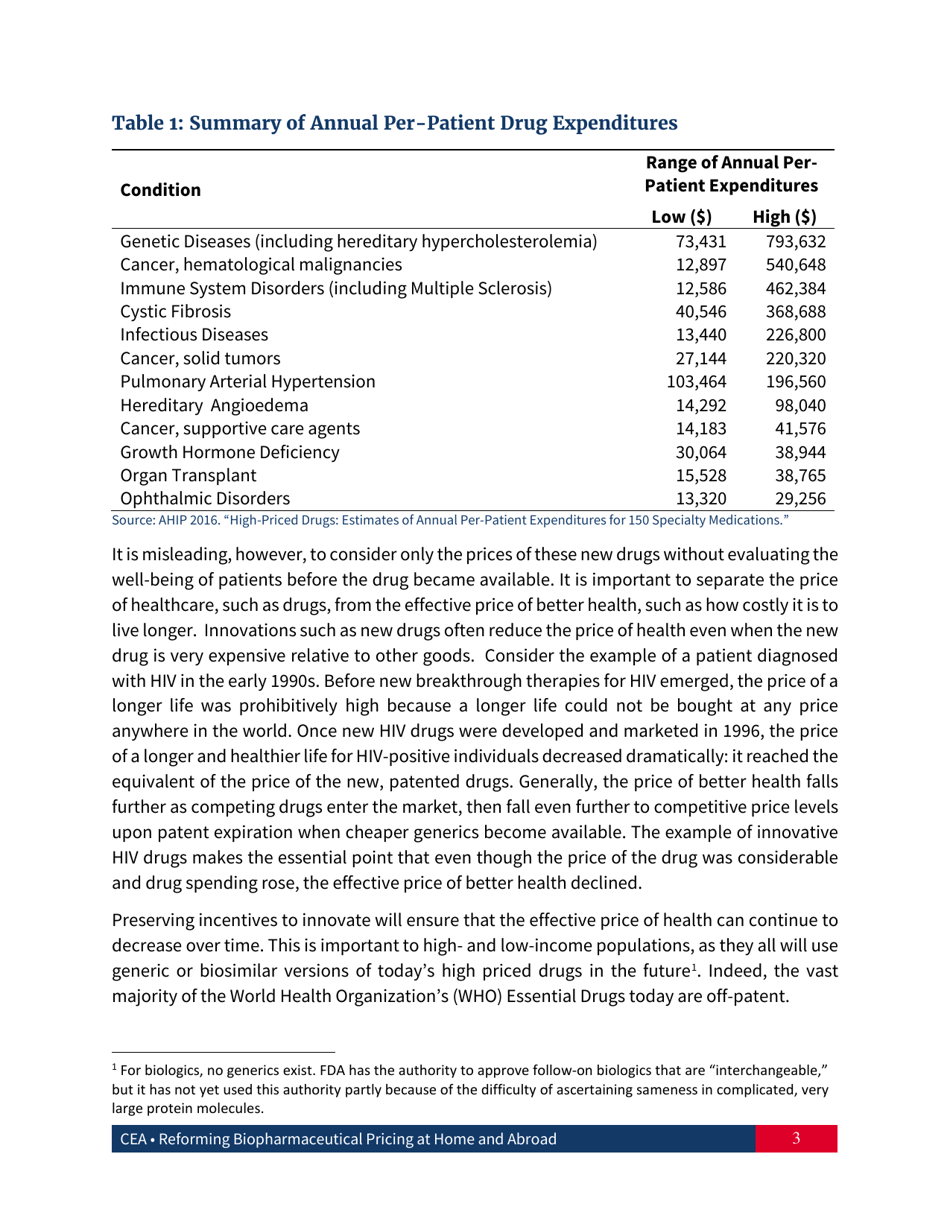
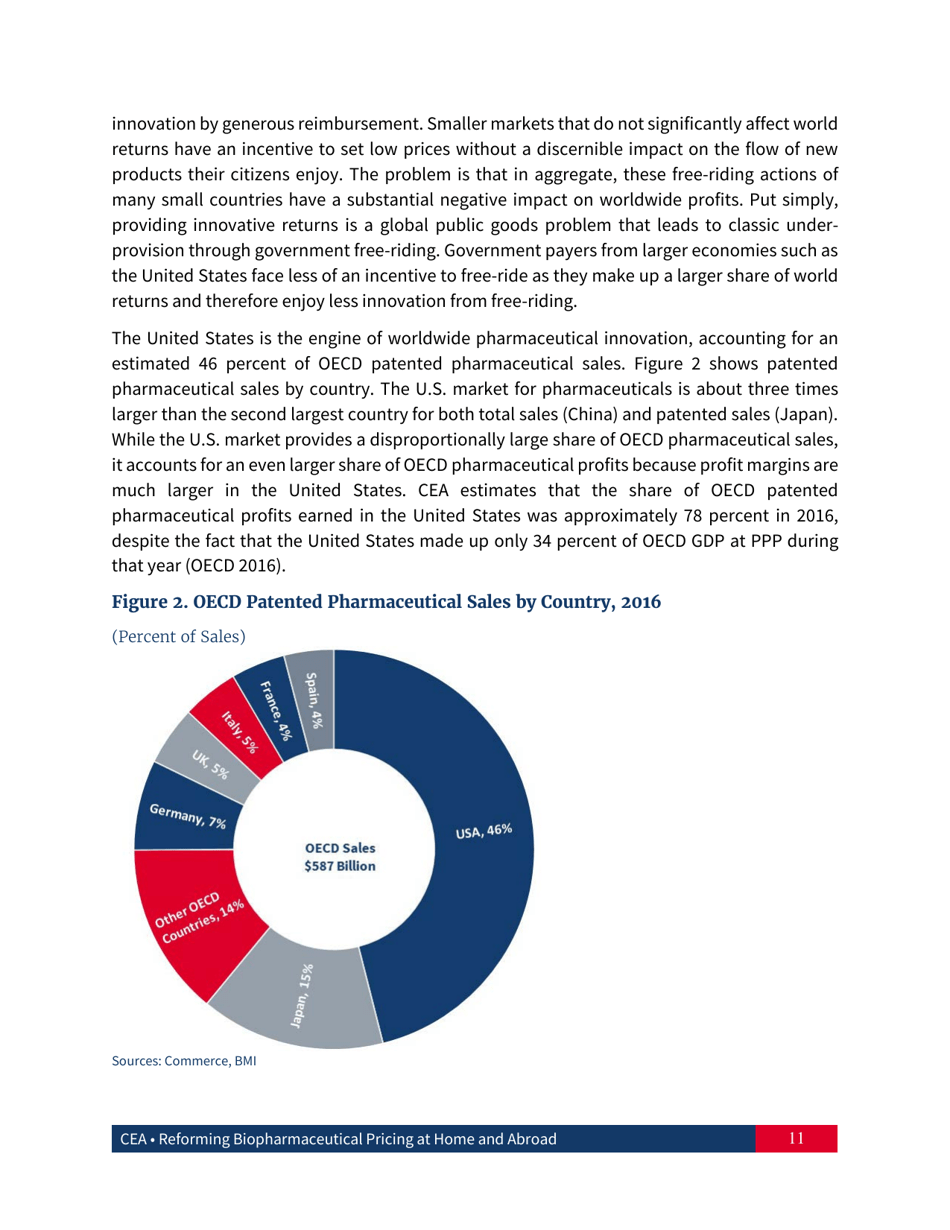
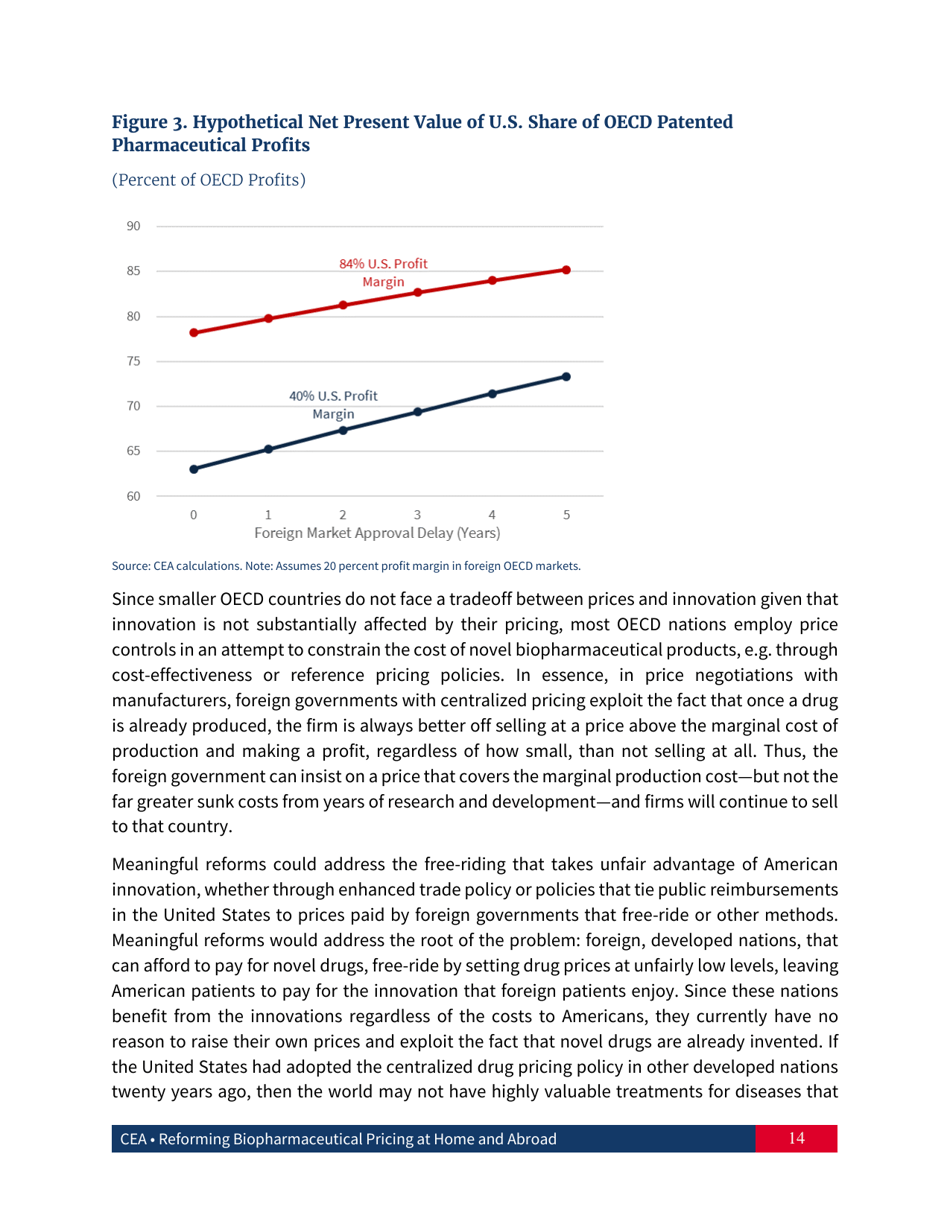
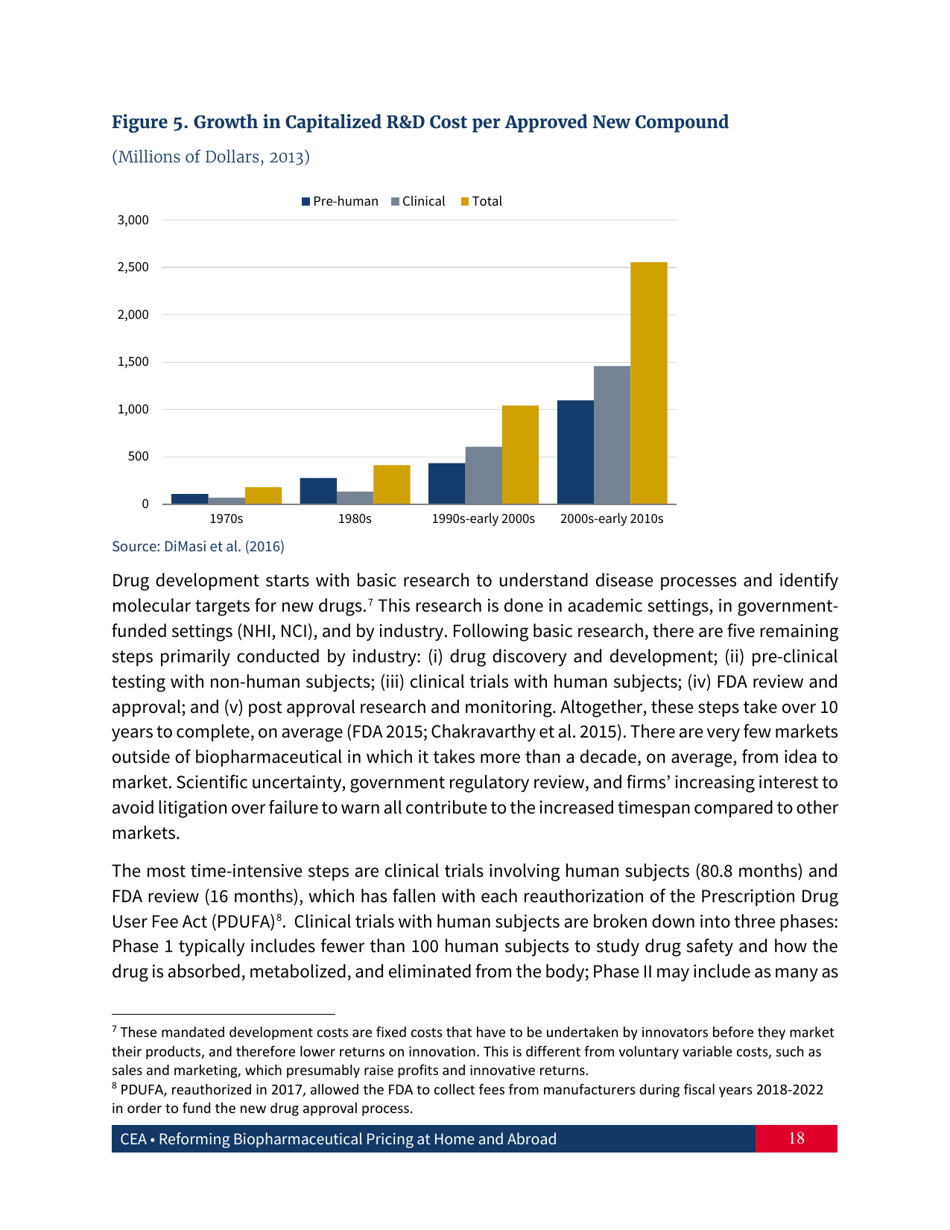
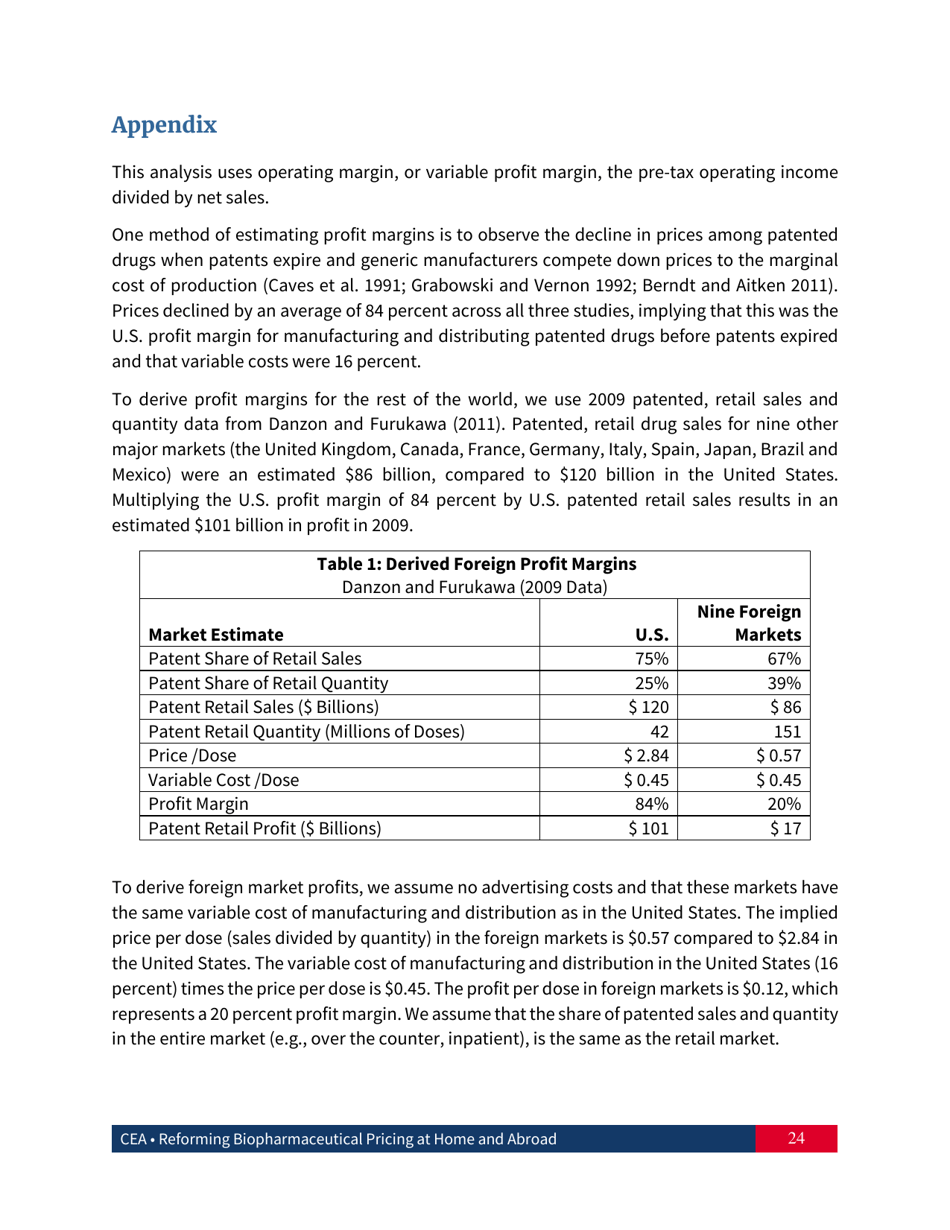
Reforming Biopharmaceutical Pricing at Home and Abroad
Reforming Biopharmaceutical Pricing at Home and Abroad is a 30-page legal document that was released by the Executive Office of the President of the United States on February 1, 2018 and used nation-wide.
FAQ
Q: What is the document about?
A: The document is about reforming biopharmaceutical pricing at home and abroad.
Q: Why is biopharmaceutical pricing being reformed?
A: Biopharmaceutical pricing is being reformed to address concerns about high drug prices and to ensure affordable access to necessary medications.
Q: Who is responsible for reforming biopharmaceutical pricing?
A: Reforming biopharmaceutical pricing involves the efforts of various stakeholders, including governments, pharmaceutical companies, and healthcare organizations.
Q: What are some of the challenges in reforming biopharmaceutical pricing?
A: Challenges in reforming biopharmaceutical pricing include balancing affordability with the need for innovation, navigating international pricing disparities, and addressing the influence of market forces.
Q: What are some potential solutions to reform biopharmaceutical pricing?
A: Potential solutions include implementing price controls, promoting competition through generic drug availability, increasing transparency in pricing, and exploring international reference pricing.
Q: How does biopharmaceutical pricing reform impact patients?
A: Biopharmaceutical pricing reform can potentially lead to more affordable medication prices, ensuring better access to necessary treatments for patients.
Q: What is the goal of biopharmaceutical pricing reform?
A: The goal of biopharmaceutical pricing reform is to strike a balance between fair pricing for innovative medications and ensuring affordability and accessibility for patients and healthcare systems.
Form Details:
- The latest edition currently provided by the Executive Office of the President of the United States;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of the form by clicking the link below or browse more legal forms and templates provided by the issuing department.