This version of the form is not currently in use and is provided for reference only. Download this version of
Form HLTH5485
for the current year.
Form HLTH5485 Special Authority Request - Adalimumab for the Treatment of Active Moderate to Severe Hidradenitis Suppurativa - British Columbia, Canada
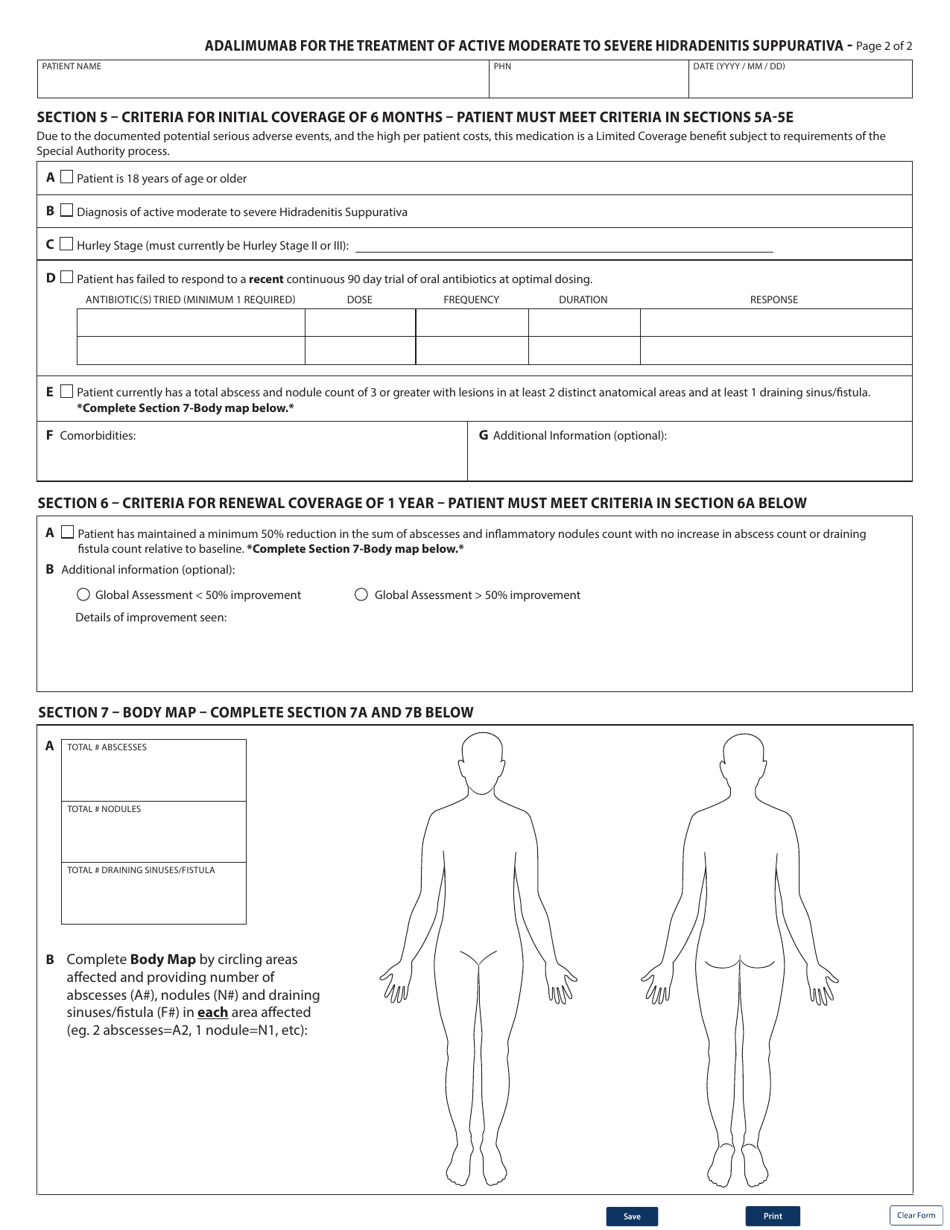
Form HLTH5485 Special Authority Request - Adalimumab for the Treatment of Active Moderate to Severe Hidradenitis Suppurativa in British Columbia, Canada is used to request special authorization for the use of the medication Adalimumab for the treatment of active moderate to severe Hidradenitis Suppurativa.
The form HLTH5485 Special Authority Request - Adalimumab for the Treatment of Active Moderate to Severe Hidradenitis Suppurativa in British Columbia, Canada is filed by the healthcare provider or the prescribing physician.
FAQ
Q: What is HLTH5485?
A: HLTH5485 is a Special Authority Request form for Adalimumab for the Treatment of Active Moderate to Severe Hidradenitis Suppurativa in British Columbia, Canada.
Q: What is Adalimumab?
A: Adalimumab is a medication used for the treatment of Hidradenitis Suppurativa.
Q: What is Hidradenitis Suppurativa?
A: Hidradenitis Suppurativa is a chronic skin condition characterized by painful, pus-filled lesions in the armpits, groin, and other areas of the body.
Q: What is a Special Authority Request?
A: A Special Authority Request is a process in which healthcare providers can apply for coverage of specific medications or treatments for their patients.
Q: Who can use HLTH5485?
A: HLTH5485 can be used by healthcare providers in British Columbia who are requesting Adalimumab for the treatment of Active Moderate to Severe Hidradenitis Suppurativa.
Q: How do I fill out the HLTH5485 form?
A: You should carefully fill out all the required sections of the HLTH5485 form, including patient information, diagnosis details, and supporting documentation.
Q: Is Adalimumab covered by insurance in British Columbia?
A: Adalimumab may be covered by insurance in British Columbia through the Special Authority Request process, depending on the patient's specific circumstances and eligibility criteria.
Q: How long does the Special Authority Request process take?
A: The processing time for a Special Authority Request can vary, but it typically takes a few weeks to receive a decision.
Q: Can Adalimumab be used for other conditions?
A: Yes, Adalimumab is also used for the treatment of other inflammatory conditions such as rheumatoid arthritis and Crohn's disease.