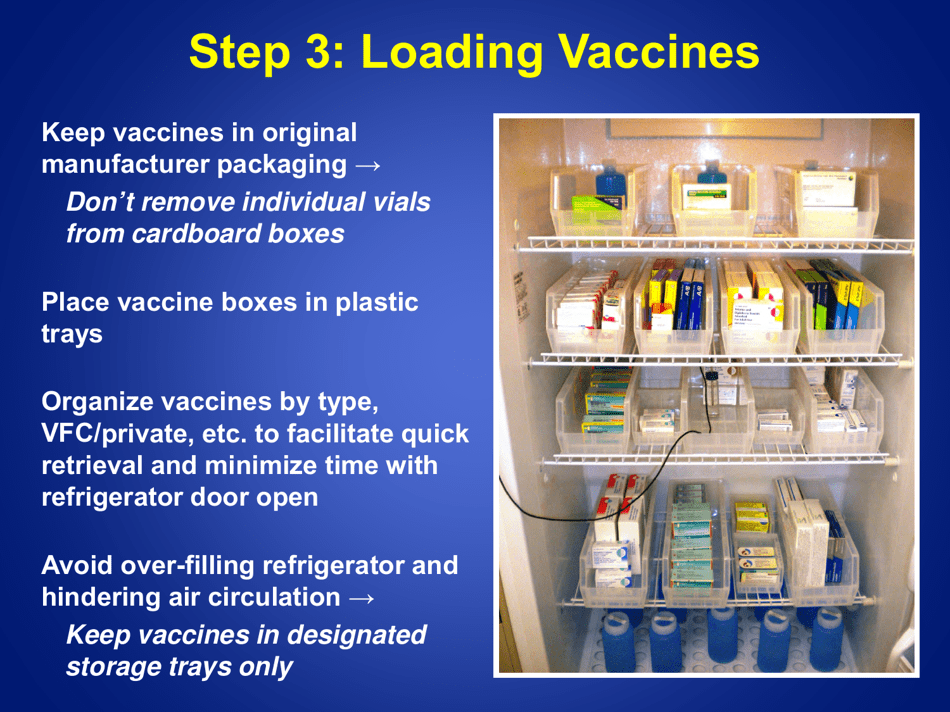
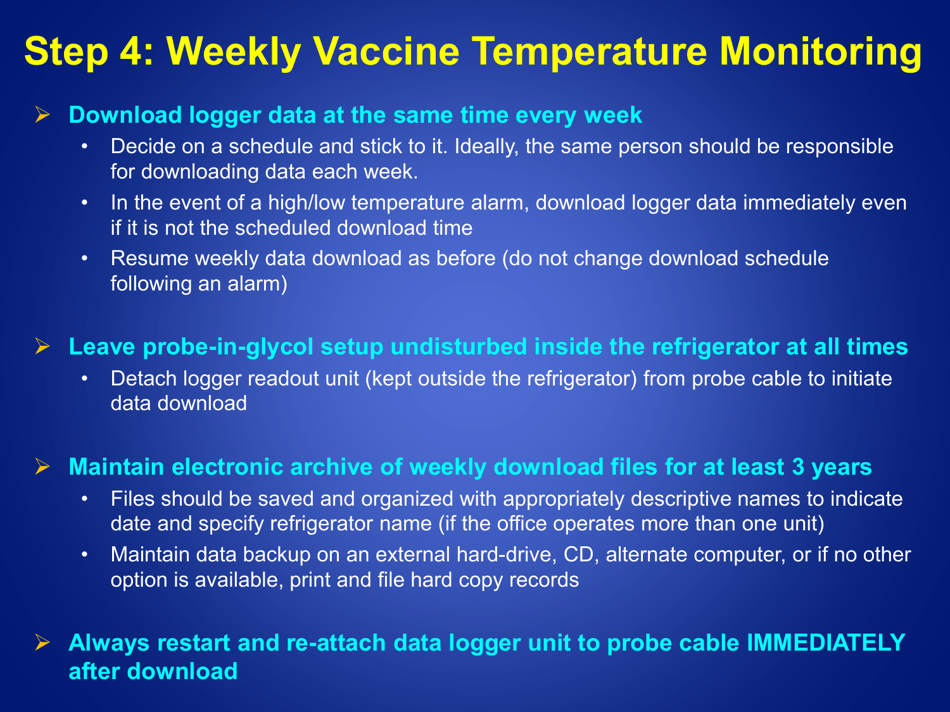
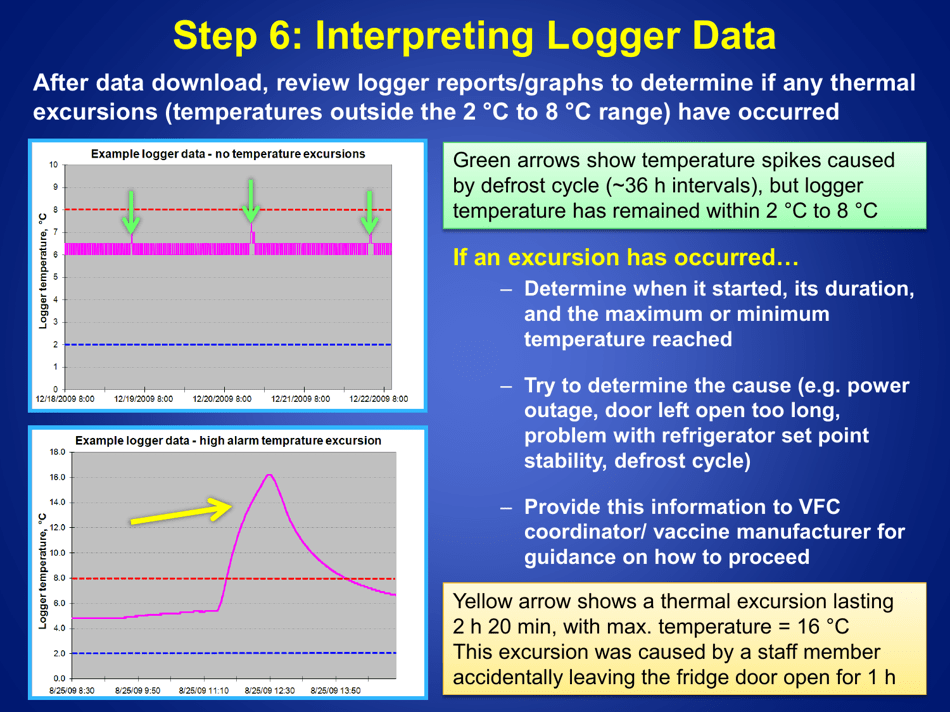
Guidelines for Storage and Temperature Monitoring of Refrigerated Vaccines
Guidelines for Storage and Temperature Monitoring of Refrigerated Vaccines is a 17-page legal document that was released by the U.S. Department of Health and Human Services - Centers for Disease Control and Prevention and used nation-wide.
FAQ
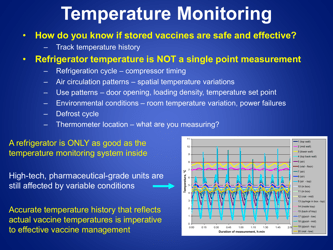
Q: Why is storage and temperature monitoring of refrigerated vaccines important?
A: Proper storage and temperature monitoring ensure the effectiveness and stability of vaccines.
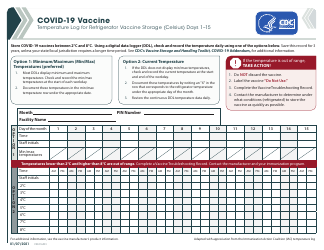
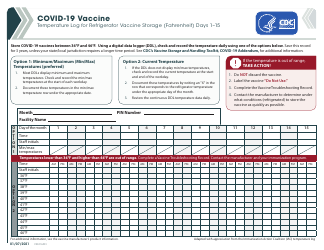
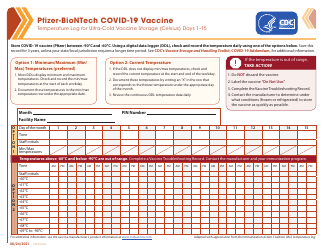
Q: What temperature range should refrigerated vaccines be kept at?
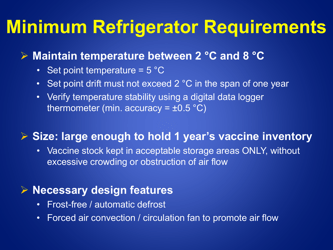
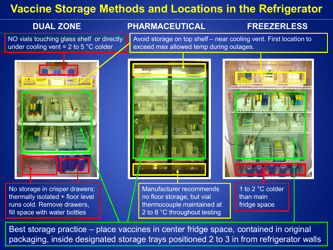
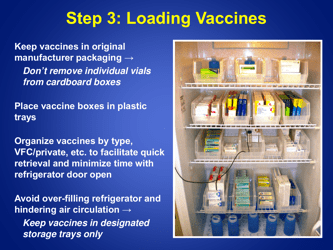
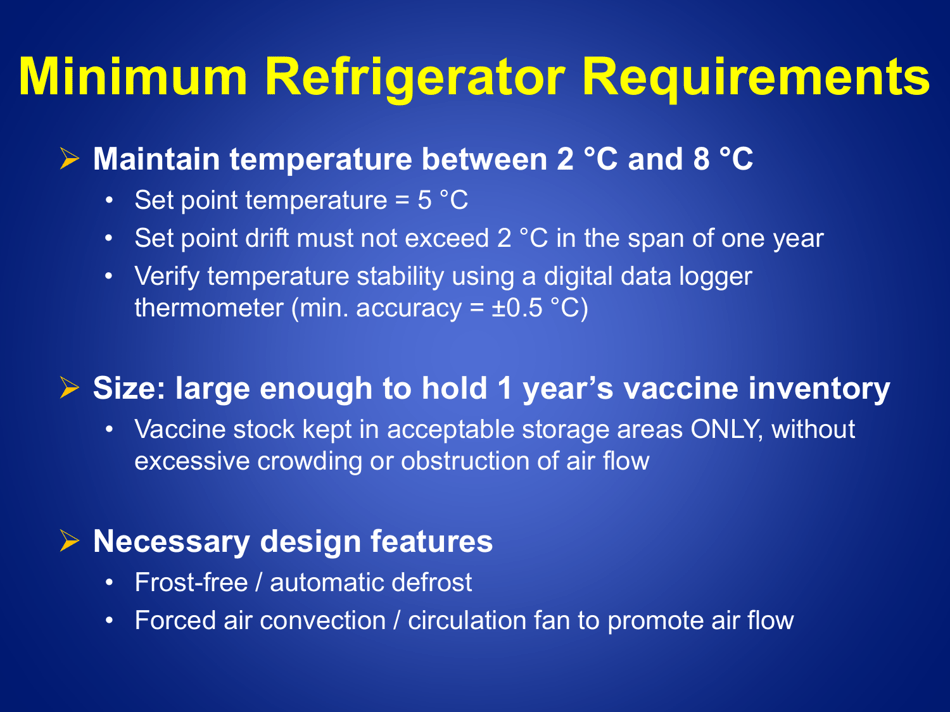
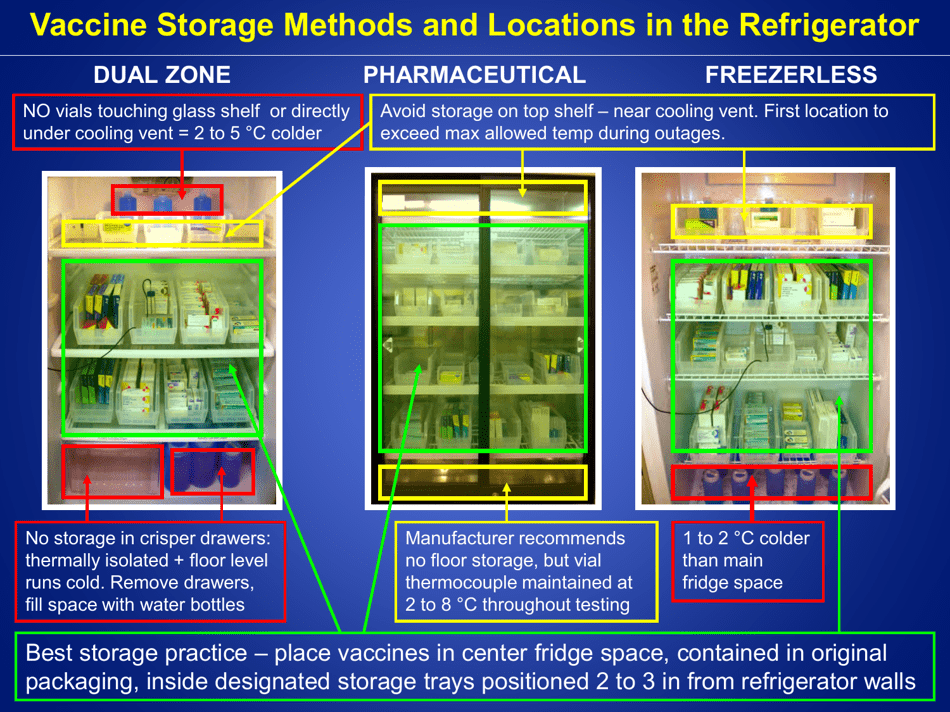
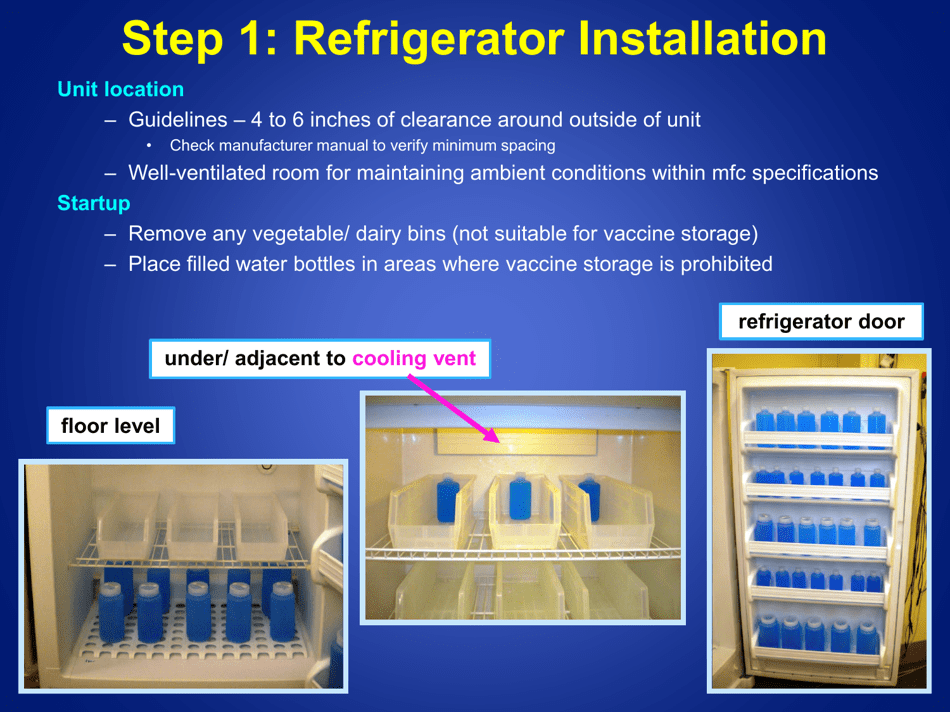
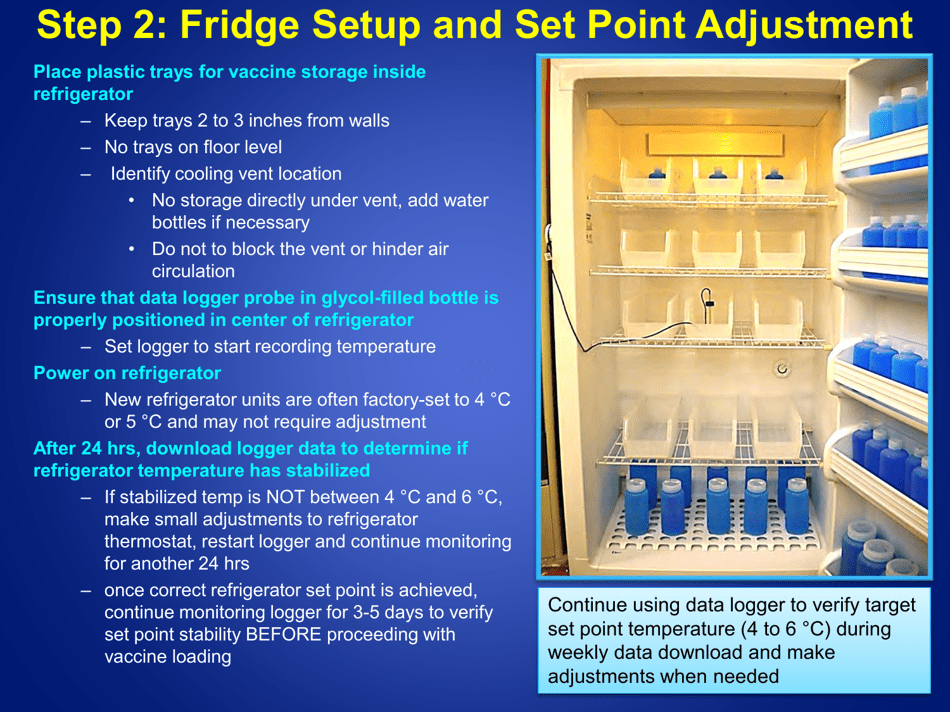
A: Refrigerated vaccines should be stored at a temperature between 2°C and 8°C (36°F and 46°F).
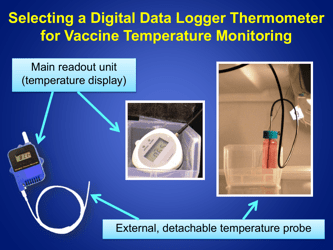
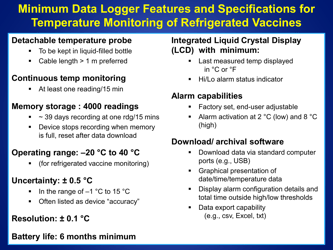
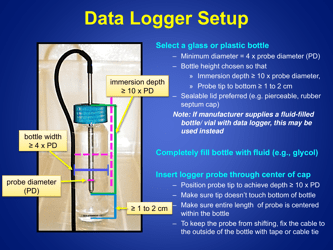
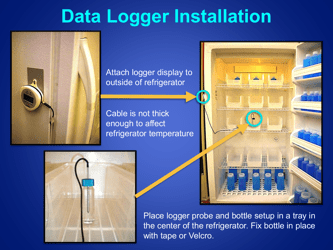
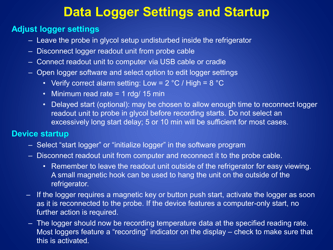
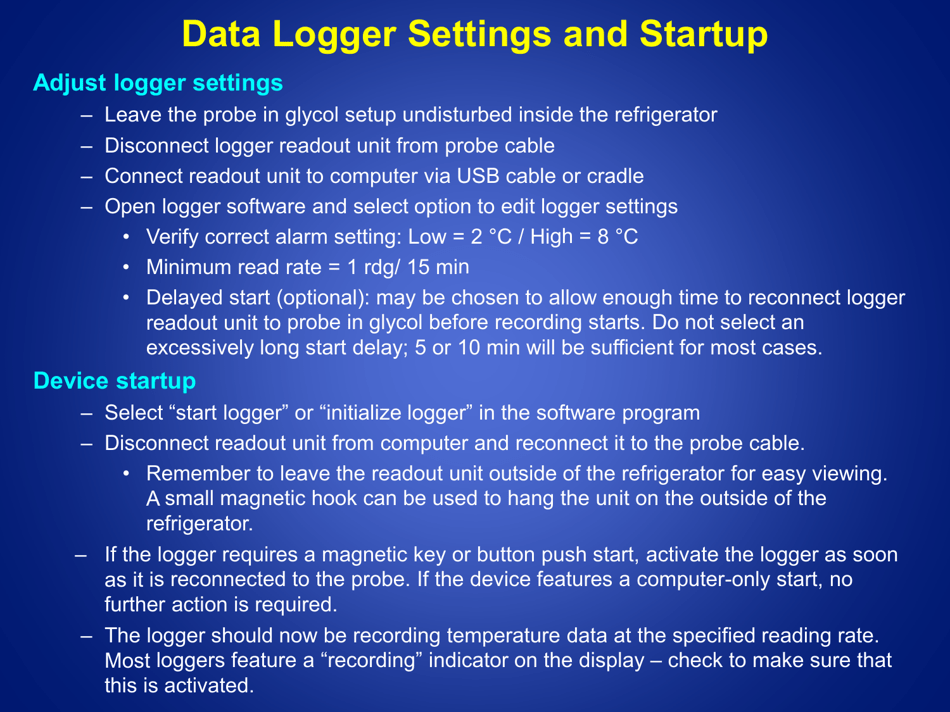
Q: What is the recommended method for temperature monitoring of refrigerated vaccines?
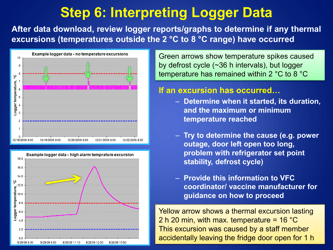
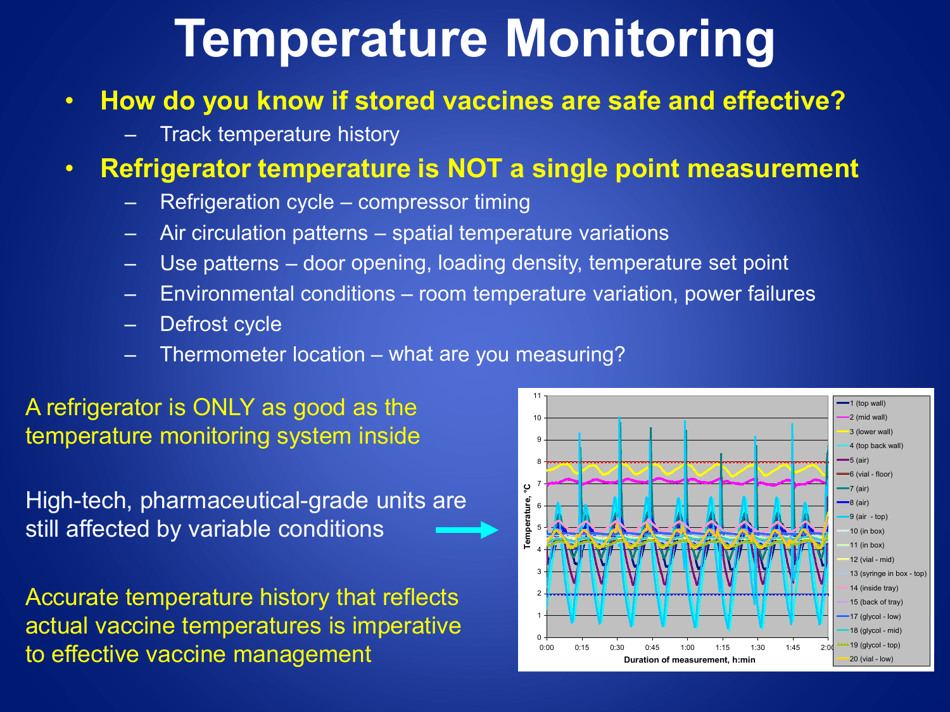
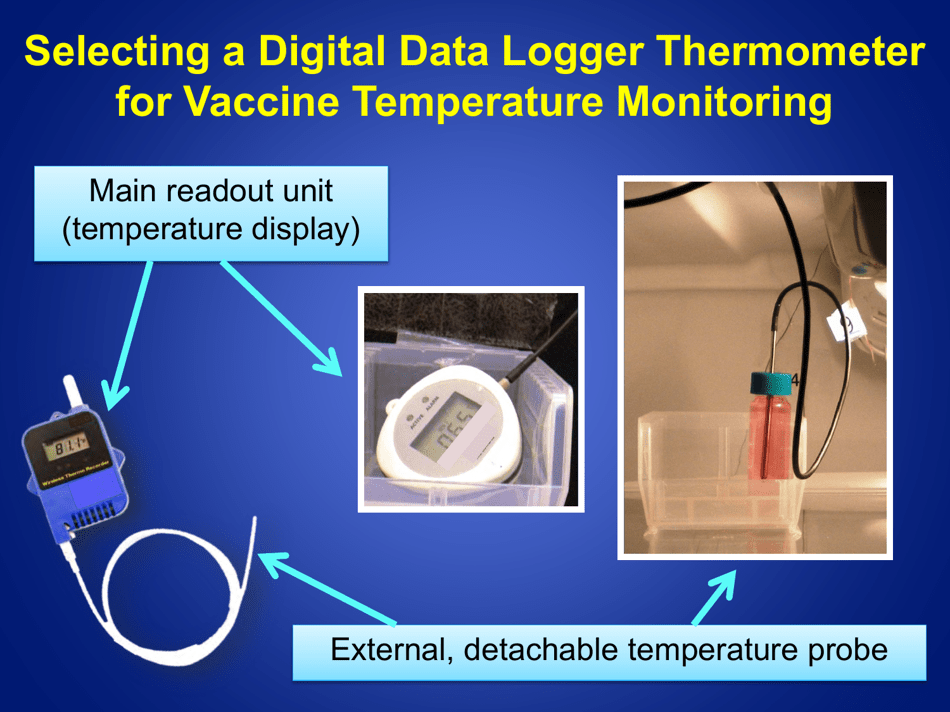
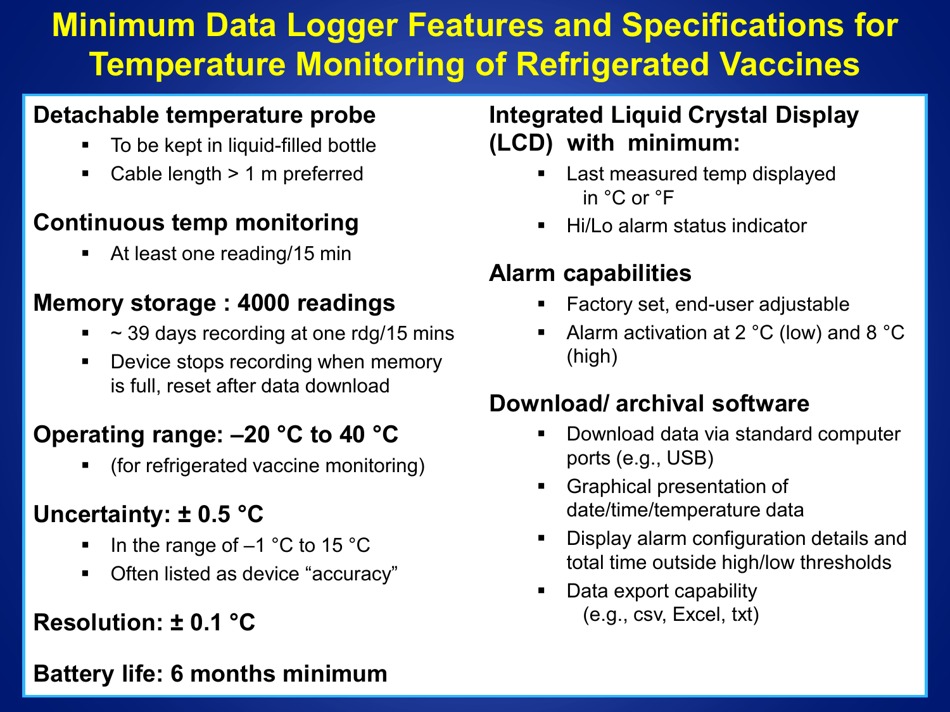
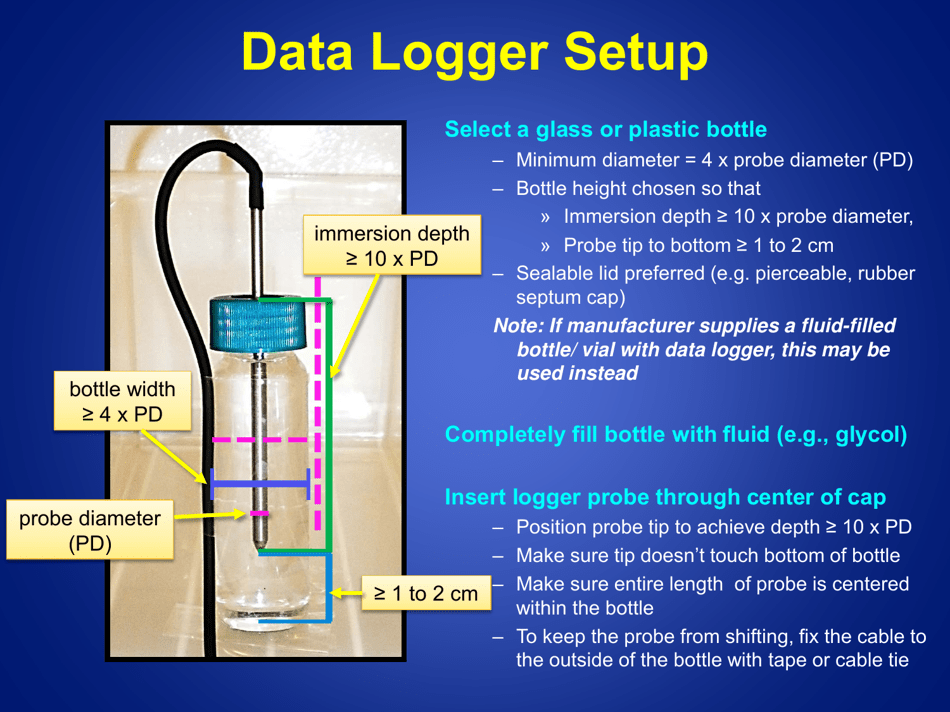
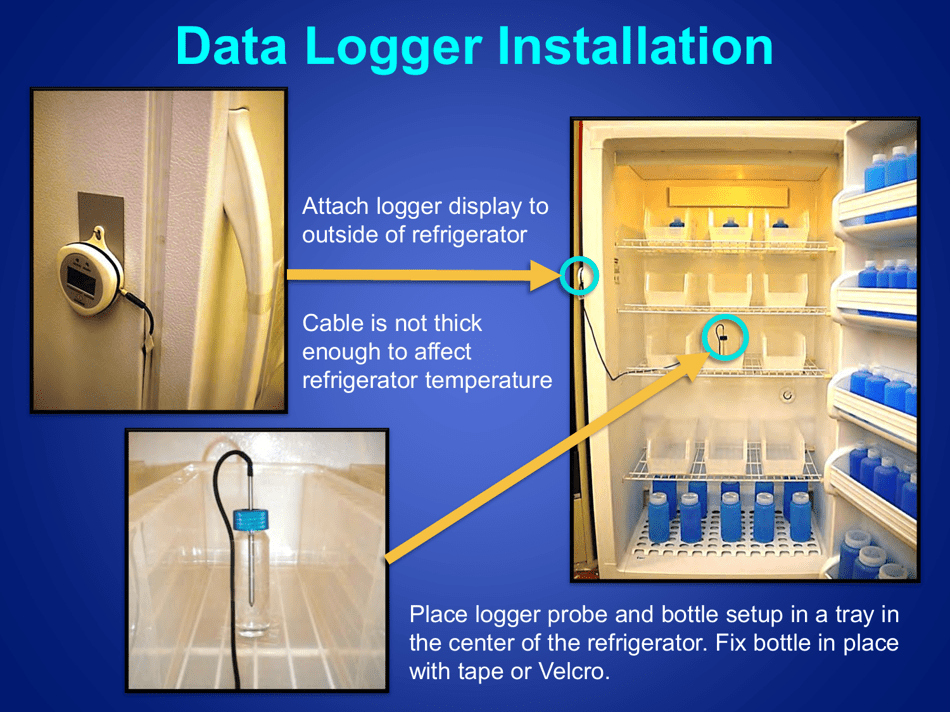
A: The recommended method is to use a calibrated temperature monitoring device, such as a data logger or a digital thermometer.
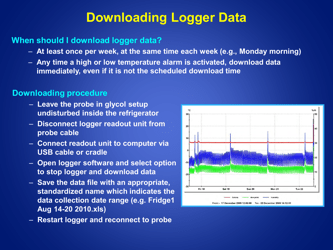
Q: How often should the temperature of refrigerated vaccines be checked?
A: The temperature of refrigerated vaccines should be checked and recorded twice daily, preferably in the morning and evening.
Q: What should be done if the temperature of the refrigerator storing vaccines goes out of the recommended range?
A: If the temperature goes out of range, the vaccines should be moved to a properly functioning refrigerator or a backup refrigerator, if available.
Q: How long can refrigerated vaccines be stored at room temperature in case of power outages?
A: Refrigerated vaccines can be stored at room temperature for a short period of time, usually up to 4 hours, during a power outage.
Form Details:
- The latest edition currently provided by the U.S. Department of Health and Human Services - Centers for Disease Control and Prevention;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of the form by clicking the link below or browse more legal forms and templates provided by the issuing department.