This version of the form is not currently in use and is provided for reference only. Download this version of
Form CMS-116
for the current year.
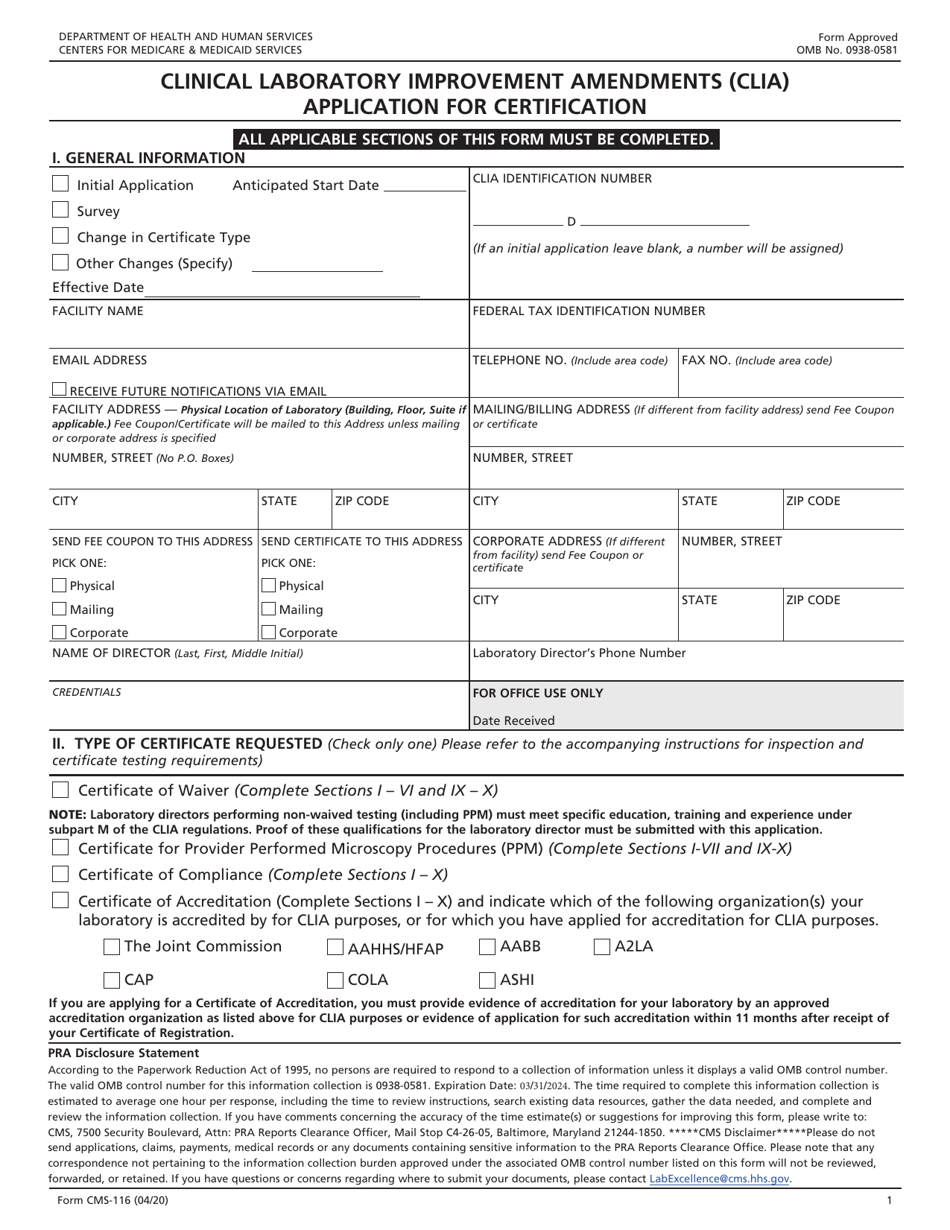
Form CMS-116 Clinical Laboratory Improvement Amendments (Clia) Application for Certification
What Is Form CMS-116?
This is a legal form that was released by the U.S. Department of Health and Human Services - Centers for Medicare and Medicaid Services on April 1, 2020 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form CMS-116?
A: Form CMS-116 is the application for certification under the Clinical Laboratory Improvement Amendments (CLIA).
Q: What is the purpose of the CLIA program?
A: The purpose of the CLIA program is to ensure quality laboratory testing is provided to all patients.
Q: Who needs to complete Form CMS-116?
A: Any laboratory seeking certification under CLIA needs to complete Form CMS-116.
Q: What information is required on Form CMS-116?
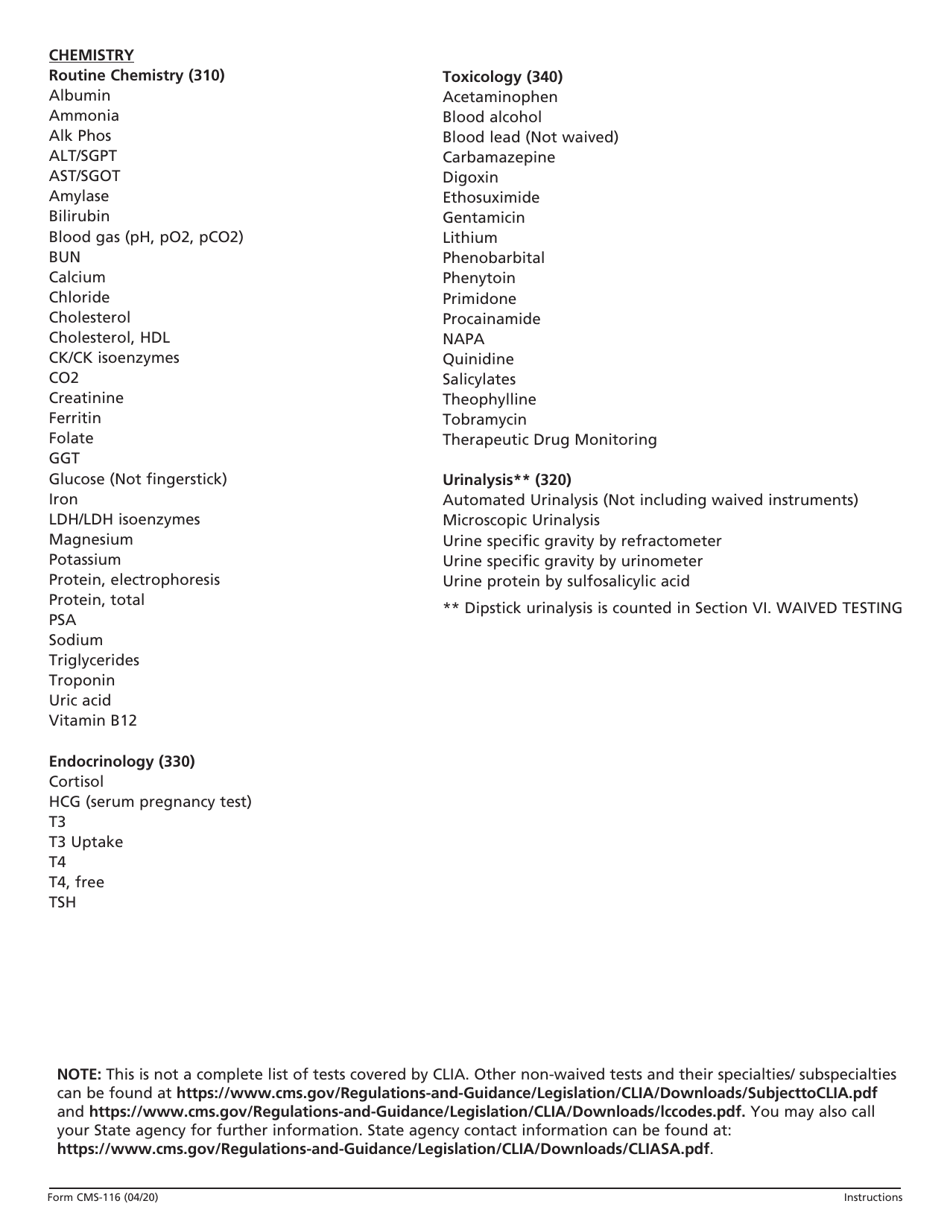
A: Form CMS-116 requires information about the laboratory's personnel, testing methods, quality control procedures, and compliance with CLIA requirements.
Q: Is there a fee for submitting Form CMS-116?
A: Yes, there is a fee associated with submitting Form CMS-116. The fee amount varies depending on the type of laboratory and the certification level being sought.
Q: How long does it take to process Form CMS-116?
A: The processing time for Form CMS-116 varies, but it typically takes several weeks to months. It is recommended to submit the application well in advance to allow for processing time.
Q: What happens after Form CMS-116 is approved?
A: After Form CMS-116 is approved, the laboratory will receive a CLIA certificate, indicating that they meet the requirements for quality laboratory testing.
Q: What if there are changes in the laboratory after Form CMS-116 is submitted?
A: If there are any changes in the laboratory, such as personnel or testing methods, the laboratory should notify the CLIA program and provide updated information.
Q: What are the consequences of operating without CLIA certification?
A: Operating without CLIA certification can result in penalties, fines, and potential closure of the laboratory.
Form Details:
- Released on April 1, 2020;
- The latest available edition released by the U.S. Department of Health and Human Services - Centers for Medicare and Medicaid Services;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form CMS-116 by clicking the link below or browse more documents and templates provided by the U.S. Department of Health and Human Services - Centers for Medicare and Medicaid Services.