This version of the form is not currently in use and is provided for reference only. Download this version of
Form HLTH5385
for the current year.
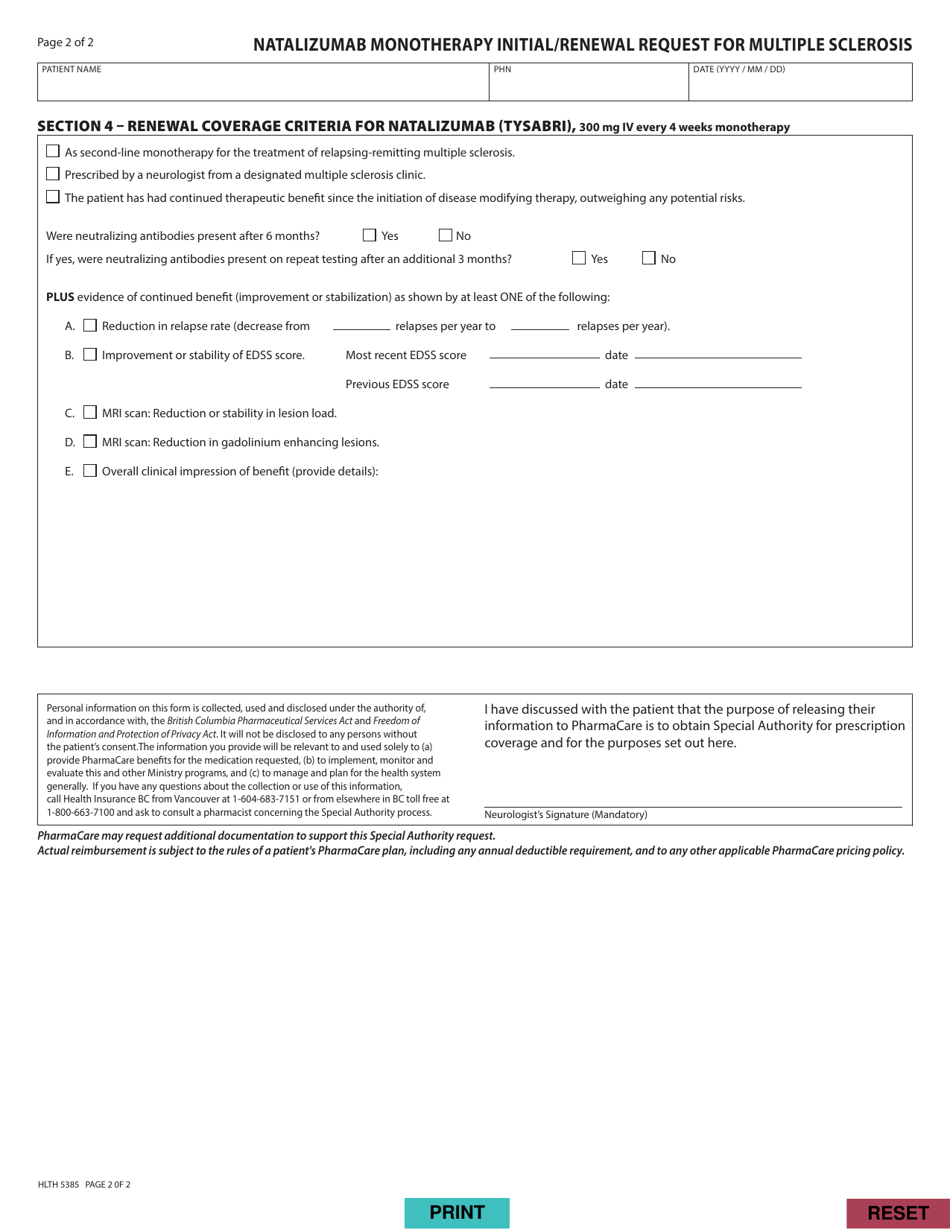
Form HLTH5385 Pharmacare Special Authority Request - Natalizumab (Tysabri) for Multiple Sclerosis - British Columbia, Canada
Form HLTH5385 Pharmacare Special Authority Request - Natalizumab (Tysabri) for Multiple Sclerosis - British Columbia, Canada is a form used for requesting special authorization for the medication Natalizumab (Tysabri) to treat Multiple Sclerosis in the province of British Columbia, Canada.
The form HLTH5385 Pharmacare Special Authority Request - Natalizumab (Tysabri) for Multiple Sclerosis in British Columbia, Canada is typically filed by the patient's prescribing physician or healthcare provider.
FAQ
Q: What is HLTH5385 Pharmacare Special Authority Request?
A: HLTH5385 Pharmacare Special Authority Request is a form used in British Columbia, Canada to request special authorization for the medication Natalizumab (Tysabri) for Multiple Sclerosis.
Q: What is Natalizumab (Tysabri)?
A: Natalizumab (Tysabri) is a medication used to treat Multiple Sclerosis.
Q: Who can use the HLTH5385 form?
A: The HLTH5385 form can be used by residents of British Columbia, Canada who require special authorization for Natalizumab (Tysabri) for the treatment of Multiple Sclerosis.
Q: How can I obtain the HLTH5385 form?
A: The HLTH5385 form can be obtained from healthcare providers or the British Columbia Ministry of Health.
Q: What is the purpose of the Special Authority Request?
A: The purpose of the Special Authority Request is to seek approval for coverage of Natalizumab (Tysabri) through the Pharmacare program for the treatment of Multiple Sclerosis.
Q: What information is required on the HLTH5385 form?
A: The HLTH5385 form requires information about the patient, prescriber, and the medical necessity for the use of Natalizumab (Tysabri) in treating Multiple Sclerosis.