This version of the form is not currently in use and is provided for reference only. Download this version of
Form CDPH8596
for the current year.
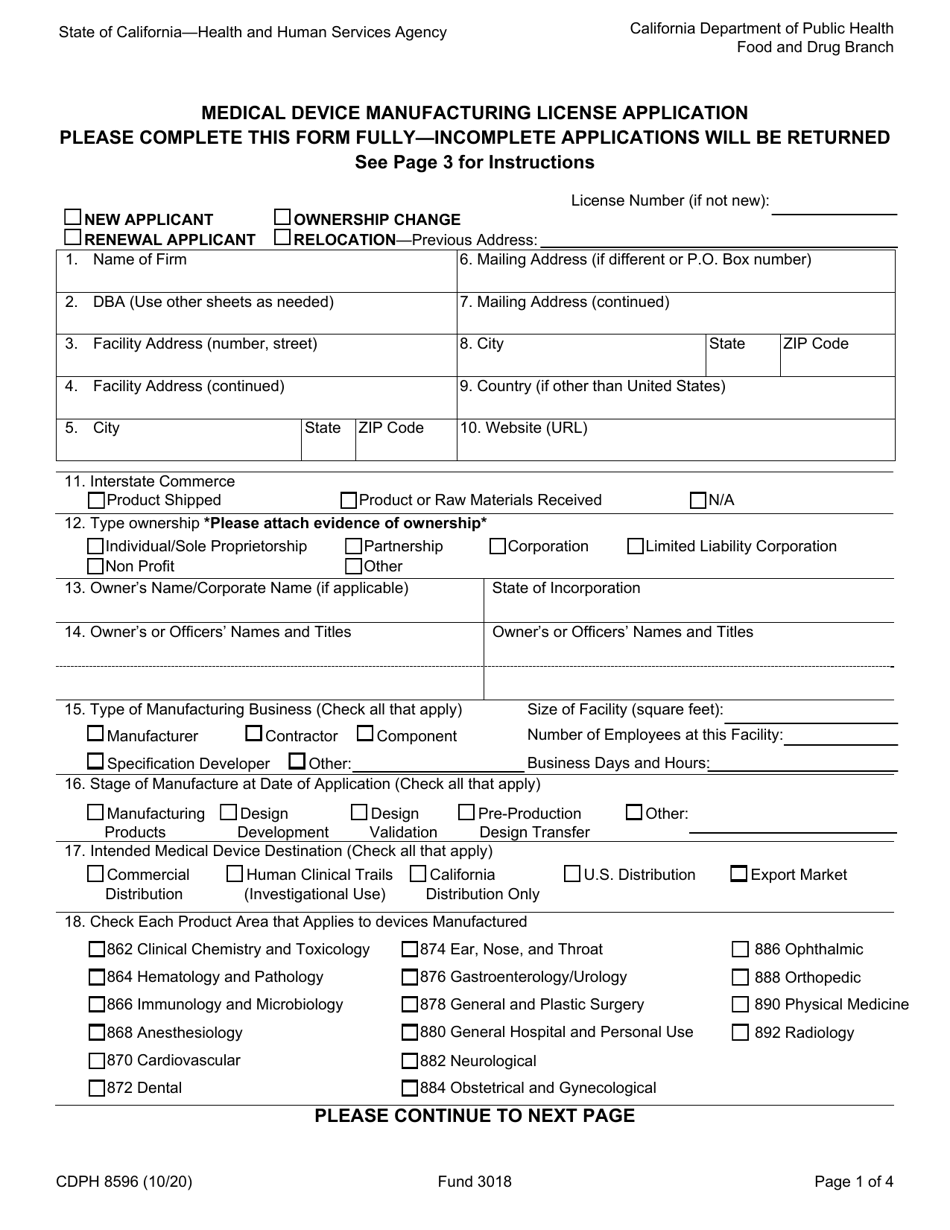
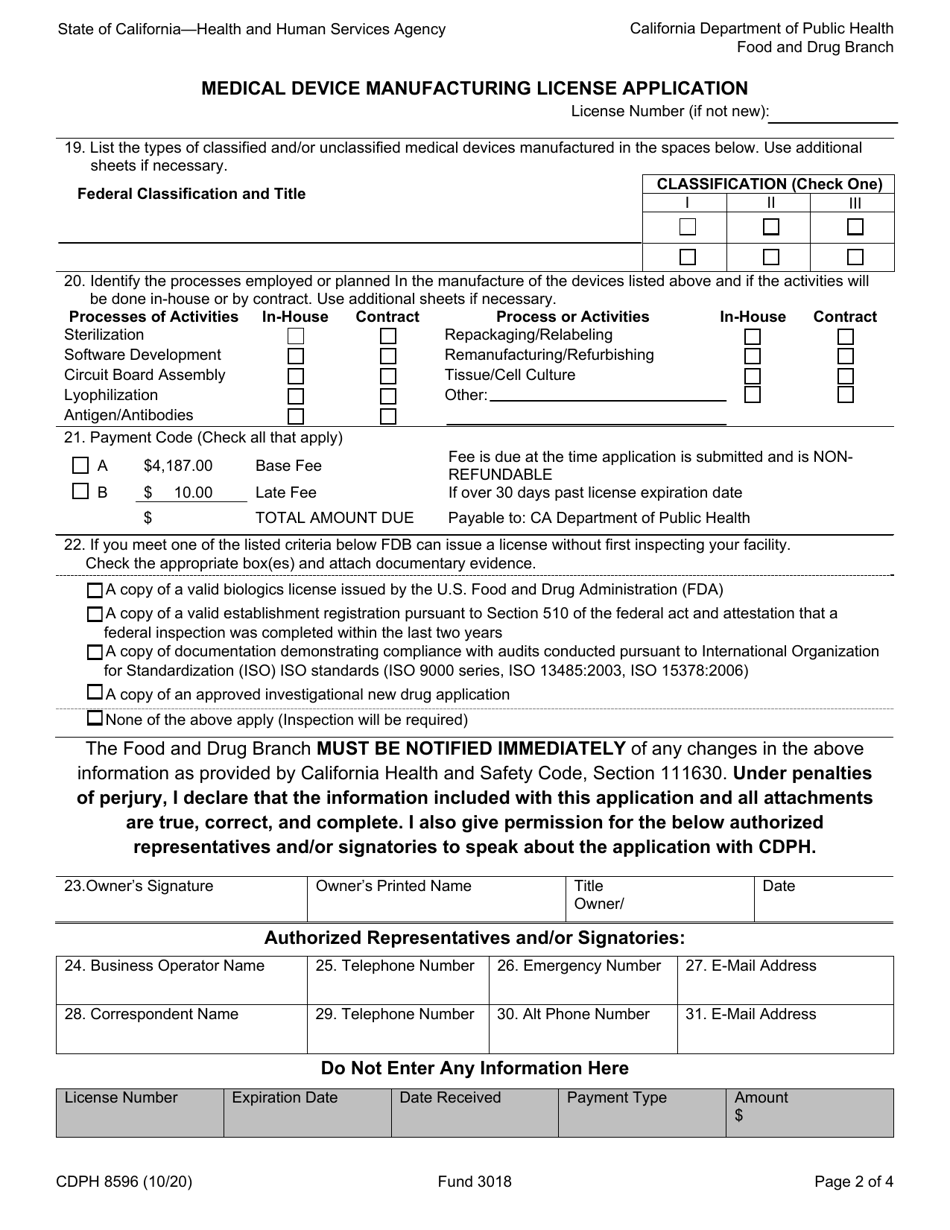
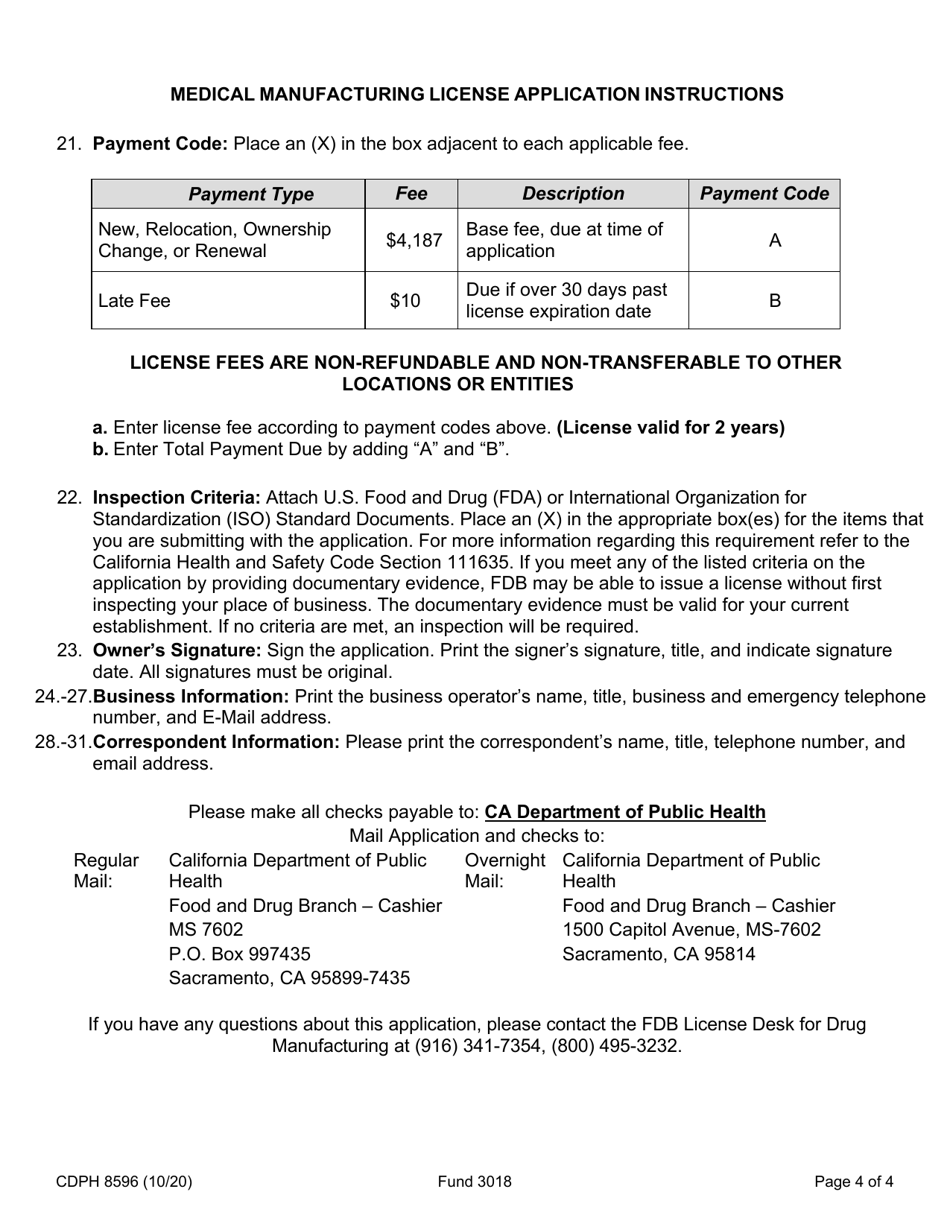
Form CDPH8596 Medical Device Manufacturing License Application - California
What Is Form CDPH8596?
This is a legal form that was released by the California Department of Public Health - a government authority operating within California. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is the CDPH8596 Medical Device Manufacturing License Application?
A: The CDPH8596 Medical Device Manufacturing License Application is a form that needs to be filled out in order to apply for a medical device manufacturing license in the state of California.
Q: What information is required on the CDPH8596 Medical Device Manufacturing License Application?
A: The CDPH8596 Medical Device Manufacturing License Application requires information such as the name and address of the manufacturing facility, a list of all medical devices manufactured, information about quality control processes, and documentation of compliance with FDA regulations.
Q: How long does it take to process the CDPH8596 Medical Device Manufacturing License Application?
A: The processing time for the CDPH8596 Medical Device Manufacturing License Application can vary but it typically takes several weeks to a few months. It is recommended to submit the application well in advance of the desired licensing date.
Q: Is the CDPH8596 Medical Device Manufacturing License Application specific to California?
A: Yes, the CDPH8596 Medical Device Manufacturing License Application is specific to the state of California. Other states may have their own application processes for medical device manufacturing licenses.
Form Details:
- Released on October 1, 2020;
- The latest edition provided by the California Department of Public Health;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form CDPH8596 by clicking the link below or browse more documents and templates provided by the California Department of Public Health.