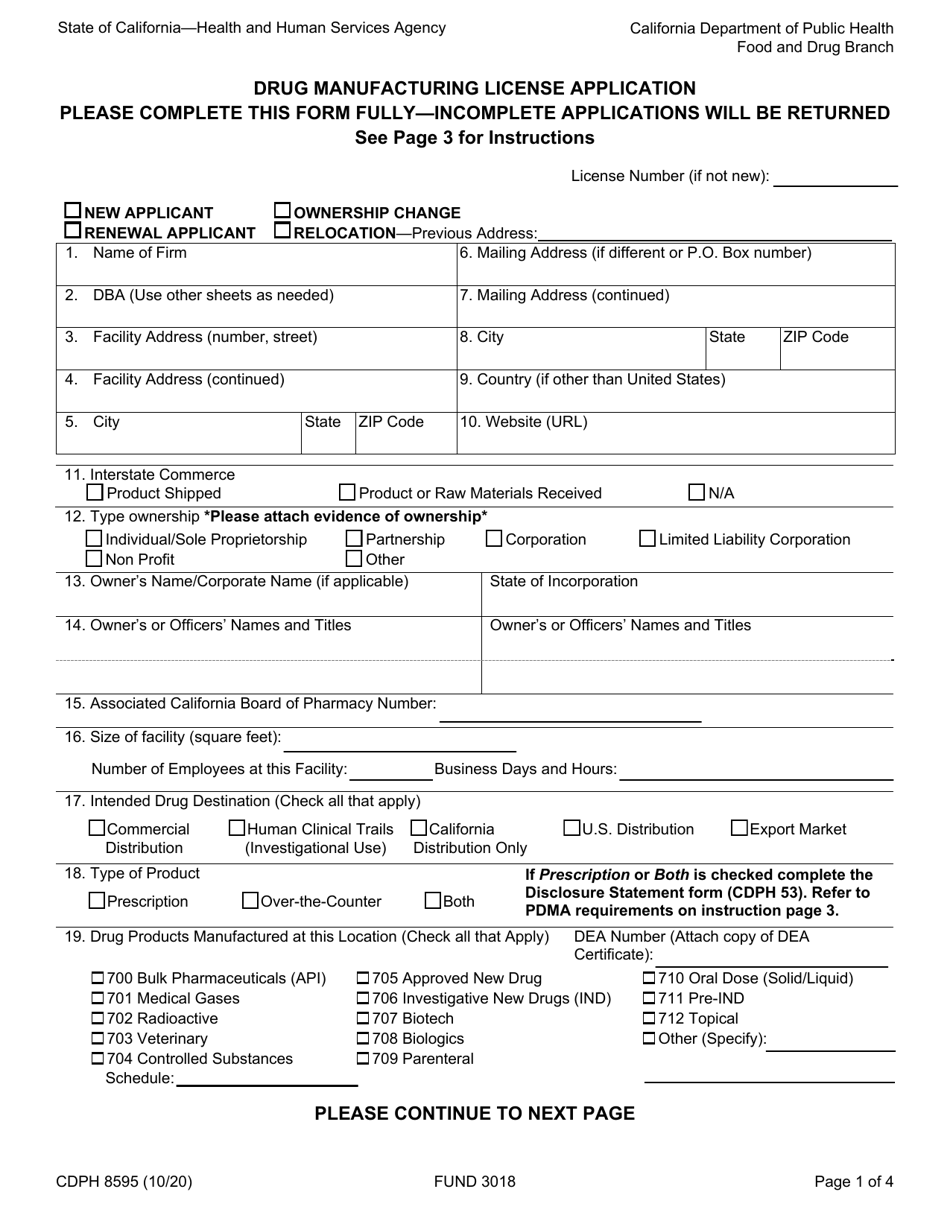
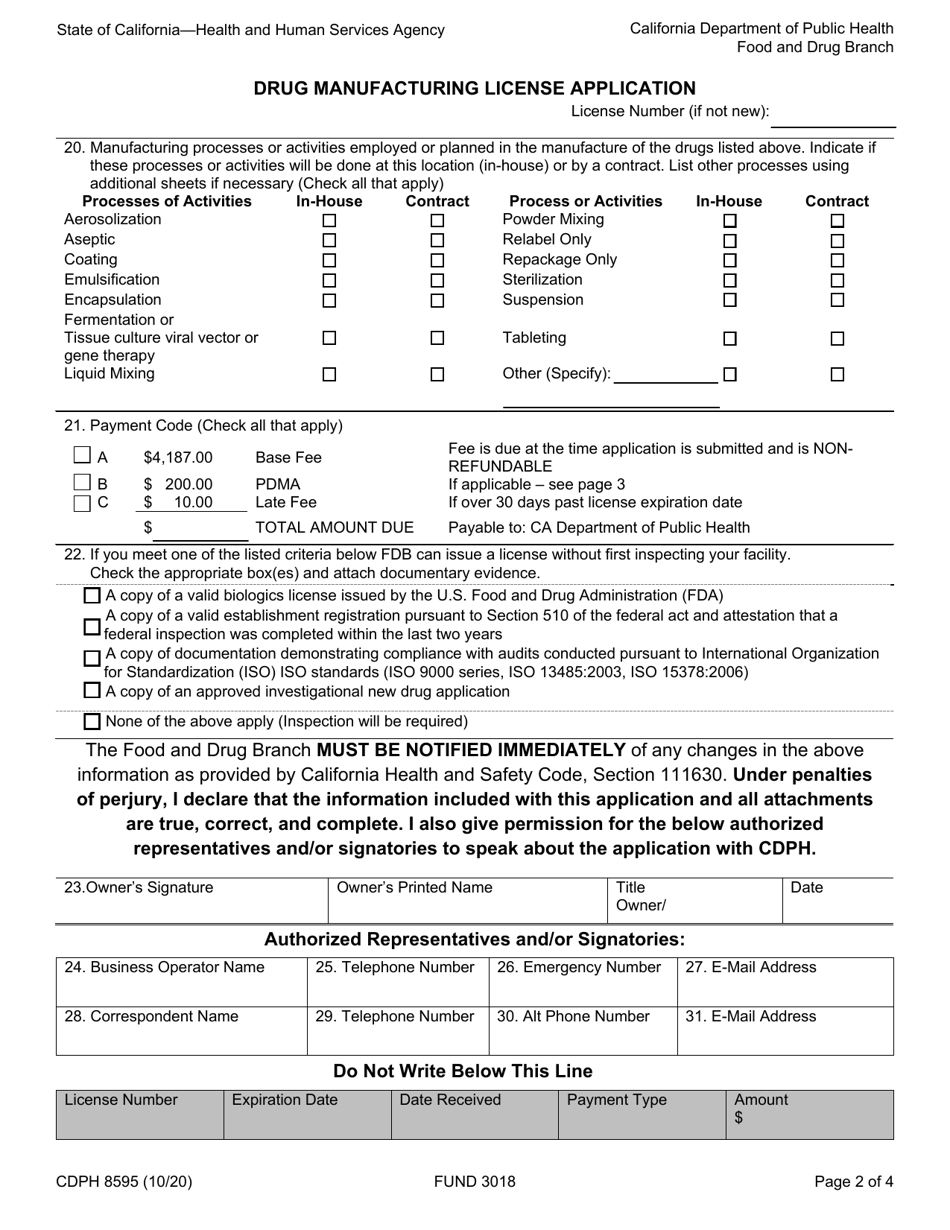
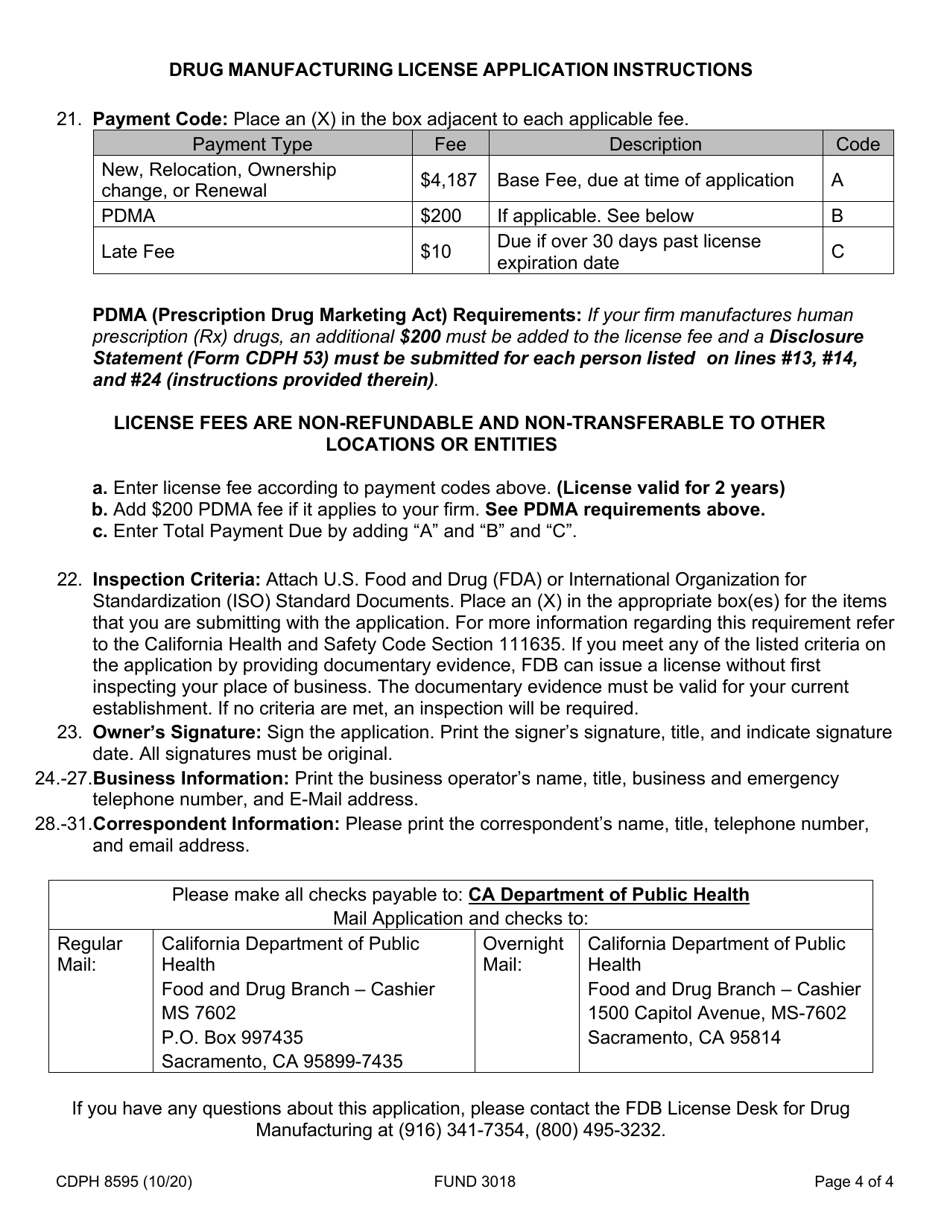
Form CDPH8595 Drug Manufacturing License Application - California
What Is Form CDPH8595?
This is a legal form that was released by the California Department of Public Health - a government authority operating within California. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is the CDPH8595 Drug Manufacturing License Application?
A: The CDPH8595 Drug Manufacturing License Application is the official application form for obtaining a drug manufacturing license in California.
Q: Who needs to fill out the CDPH8595 Drug Manufacturing License Application?
A: Any individual or entity that wants to engage in drug manufacturing in California needs to fill out the CDPH8595 application.
Q: What information is required on the CDPH8595 Drug Manufacturing License Application?
A: The CDPH8595 application requires information such as the applicant's name, business details, manufacturing processes, quality control measures, and compliance with regulations.
Q: Is there a deadline for submitting the CDPH8595 Drug Manufacturing License Application?
A: There is no specific deadline for submitting the application, but it is recommended to submit it as soon as possible to allow for processing and evaluation.
Q: How long does it take to process the CDPH8595 Drug Manufacturing License Application?
A: The processing time for the application can vary. It is advisable to check with the CDPH for the most current information on processing times.
Q: What happens after submitting the CDPH8595 Drug Manufacturing License Application?
A: After submitting the application, the CDPH will review the information provided and conduct inspections to ensure compliance with regulations. If approved, a drug manufacturing license will be issued.
Q: What if my CDPH8595 Drug Manufacturing License Application is denied?
A: If your application is denied, you will receive notification from the CDPH outlining the reasons for denial. You may have the option to appeal the decision or reapply with corrected information.
Q: Can I start drug manufacturing before obtaining the CDPH8595 Drug Manufacturing License?
A: No, it is illegal to engage in drug manufacturing in California without a valid CDPH8595 Drug Manufacturing License. You must wait for the license to be issued before starting operations.
Form Details:
- Released on October 1, 2020;
- The latest edition provided by the California Department of Public Health;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form CDPH8595 by clicking the link below or browse more documents and templates provided by the California Department of Public Health.