Pan-Canadian Reportable Information Required for Sars-Cov-2 Animal Testing - British Columbia, Canada
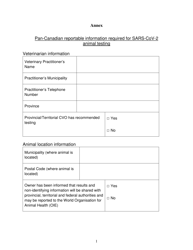
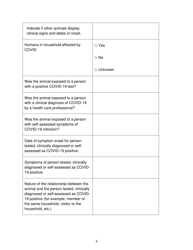
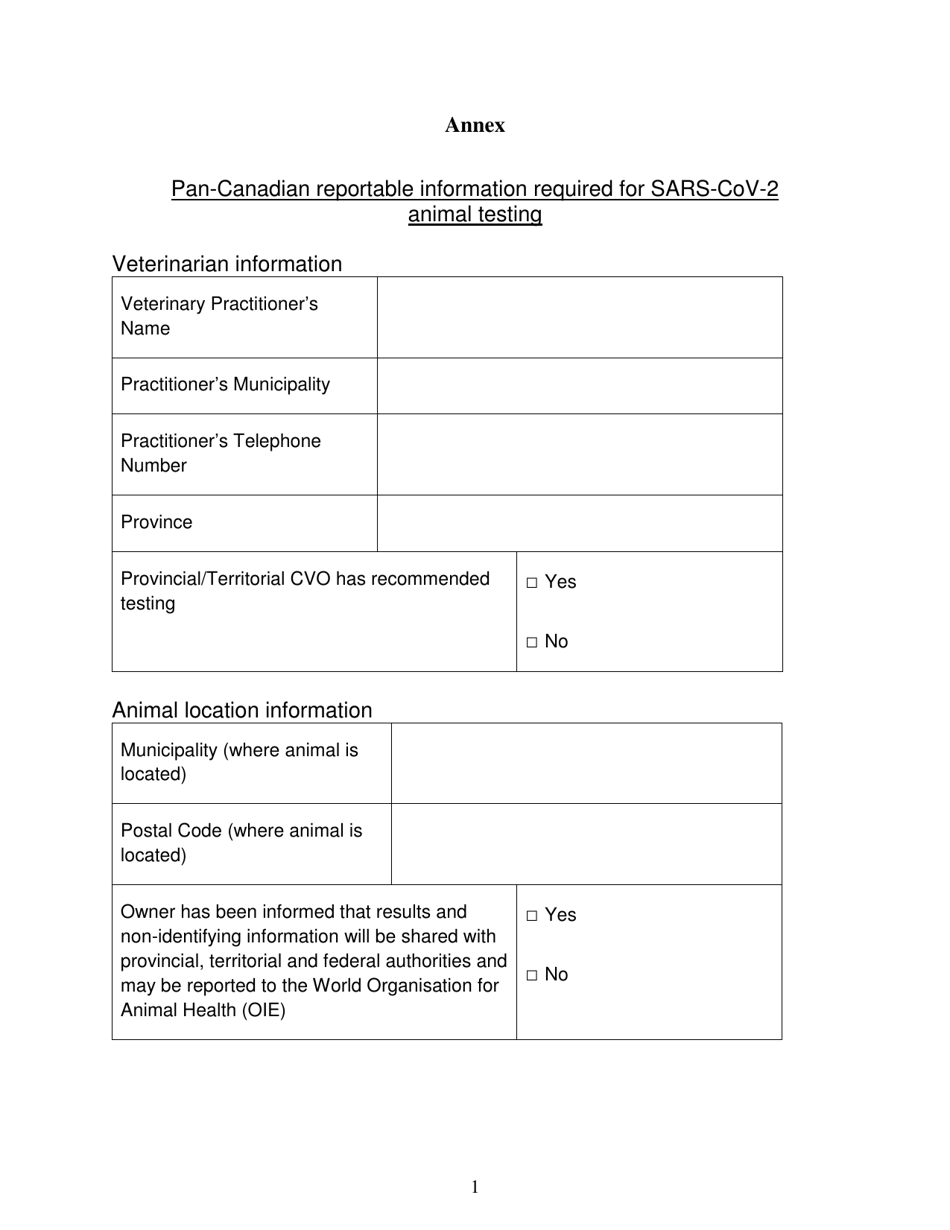
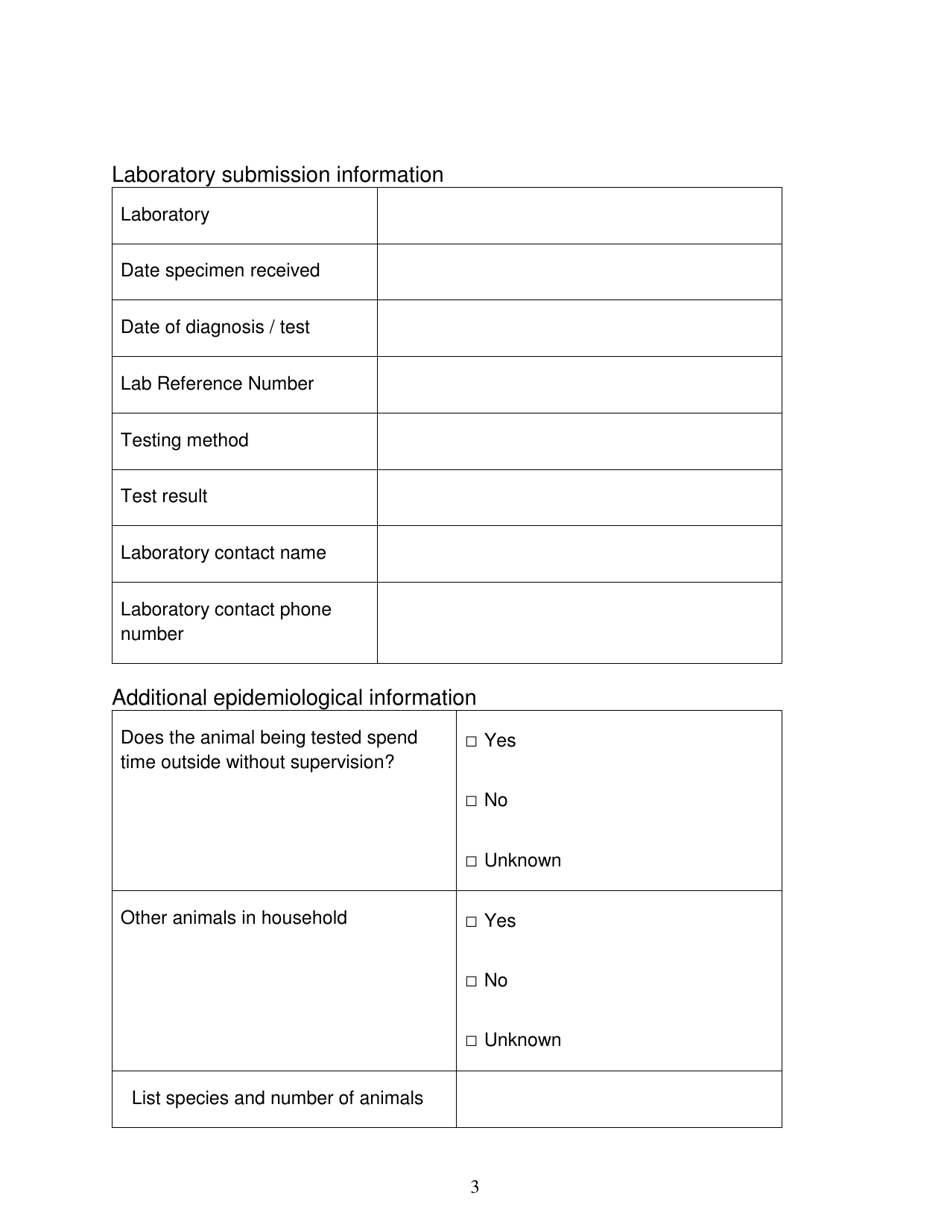
The Pan-Canadian Reportable Information Required for Sars-Cov-2 Animal Testing in British Columbia, Canada is a document that outlines the specific information that needs to be reported when conducting animal testing for detecting the Sars-Cov-2 virus. It helps in collecting and analyzing data related to animal testing for COVID-19 in order to track and understand the spread of the virus.
The Pan-Canadian reportable information required for SARS-CoV-2 animal testing in British Columbia, Canada, is likely filed by the appropriate government health authorities or agencies responsible for animal health surveillance and reporting. However, specific details about the reporting process and responsible entities should be obtained from the relevant provincial or federal authorities.
FAQ
Q: What is the purpose of the Pan-Canadian Reportable Information for SARS-CoV-2 Animal Testing?
A: The purpose is to collect and report data on SARS-CoV-2 animal testing in British Columbia, Canada.
Q: Who is responsible for reporting this information?
A: Veterinarians and laboratories conducting SARS-CoV-2 animal testing are responsible for reporting this information.
Q: Which province in Canada does this document pertain to?
A: This document pertains to British Columbia, Canada.
Q: What type of information needs to be reported?
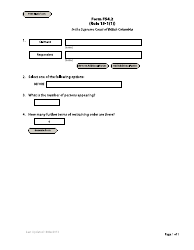
A: Information such as the number and type of animals tested, test results, and the presence of any clinical signs or symptoms in the animals must be reported.
Q: Why is it important to collect this information?
A: Collecting this information helps to monitor the spread of SARS-CoV-2 in animals, assess potential risks to human health, and inform public health measures.