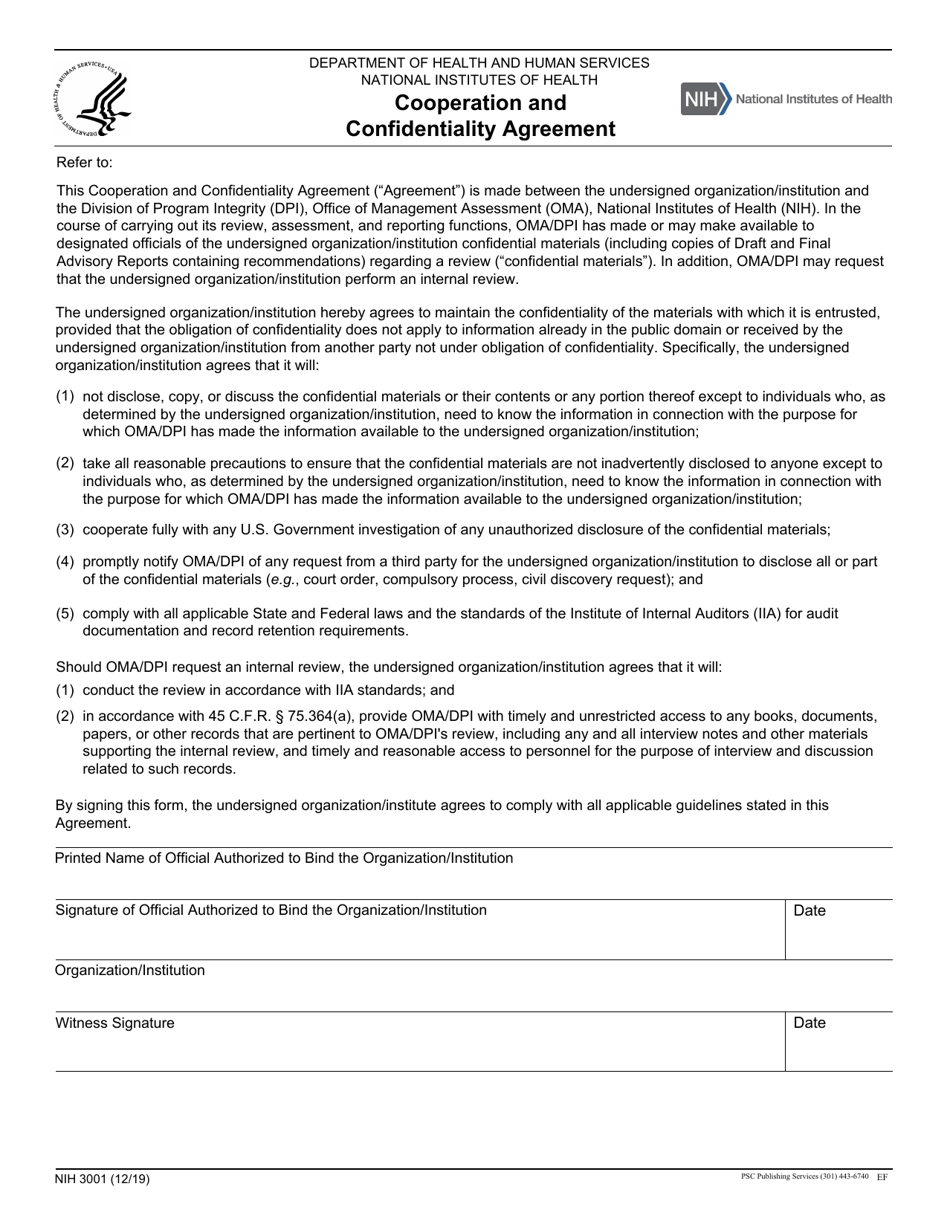
Form NIH3001 Cooperation and Confidentiality Agreement
What Is Form NIH3001?
This is a legal form that was released by the U.S. Department of Health and Human Services - National Institutes of Health on December 1, 2019 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is the purpose of the NIH3001 Cooperation and Confidentiality Agreement?
A: The purpose of the NIH3001 Cooperation and Confidentiality Agreement is to outline the terms of cooperation and confidentiality between parties involved in a project.
Q: Who needs to sign the NIH3001 Cooperation and Confidentiality Agreement?
A: Anyone involved in the project who will have access to confidential information or will be collaborating with others on the project needs to sign the agreement.
Q: What does the NIH3001 Cooperation and Confidentiality Agreement cover?
A: The agreement covers topics such as the scope of cooperation, confidentiality of information, use of information, intellectual property rights, and dispute resolution.
Q: Can the NIH3001 Cooperation and Confidentiality Agreement be modified?
A: Yes, the agreement can be modified, but any modifications need to be agreed upon in writing by all parties involved.
Q: Is the NIH3001 Cooperation and Confidentiality Agreement legally binding?
A: Yes, the agreement is legally binding once all parties have signed it.
Q: What happens if someone violates the terms of the NIH3001 Cooperation and Confidentiality Agreement?
A: If someone violates the terms of the agreement, there may be legal consequences, including the possibility of financial damages.
Form Details:
- Released on December 1, 2019;
- The latest available edition released by the U.S. Department of Health and Human Services - National Institutes of Health;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form NIH3001 by clicking the link below or browse more documents and templates provided by the U.S. Department of Health and Human Services - National Institutes of Health.