Sponsor Attestation Checklist for Abbreviated New Drug Submissions (Andss) - Canada
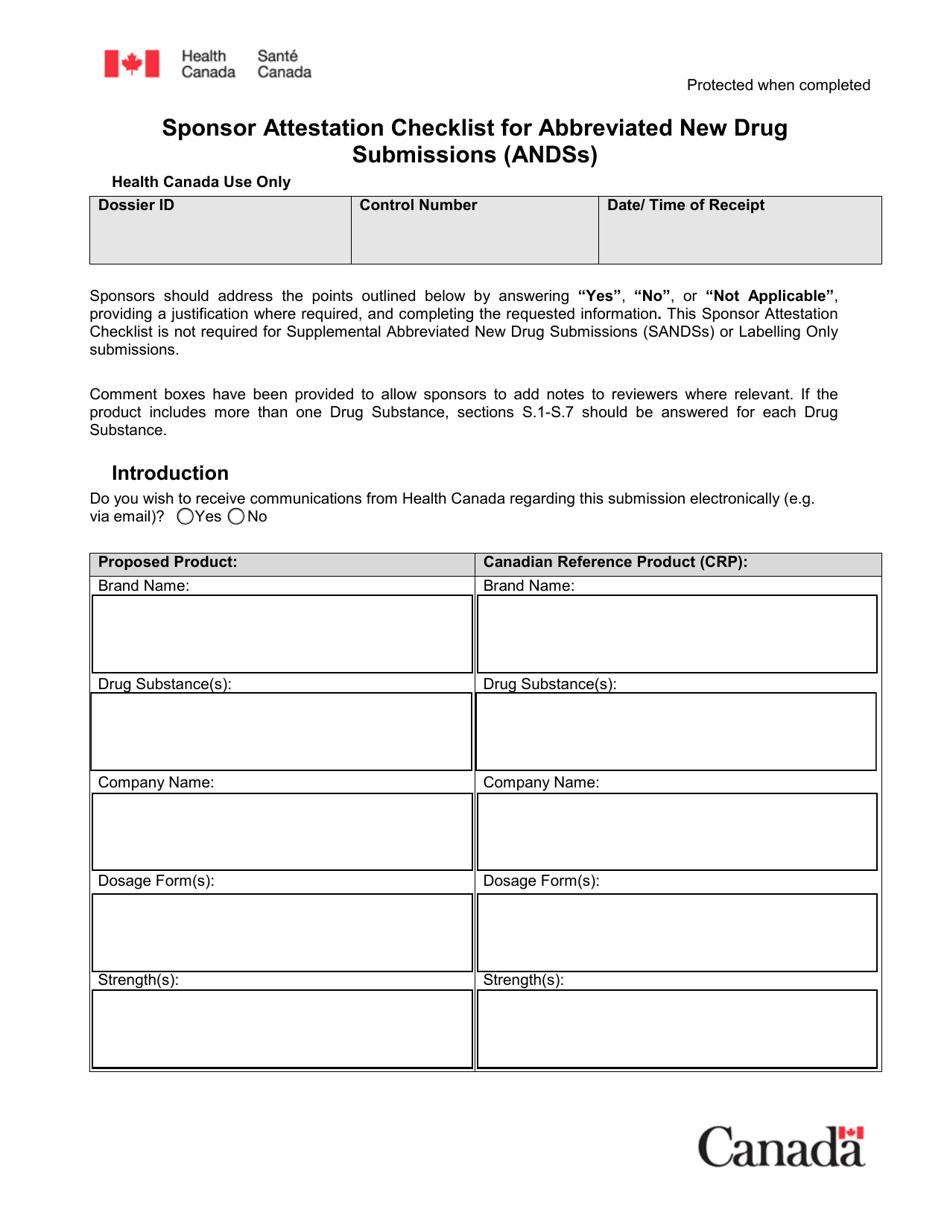
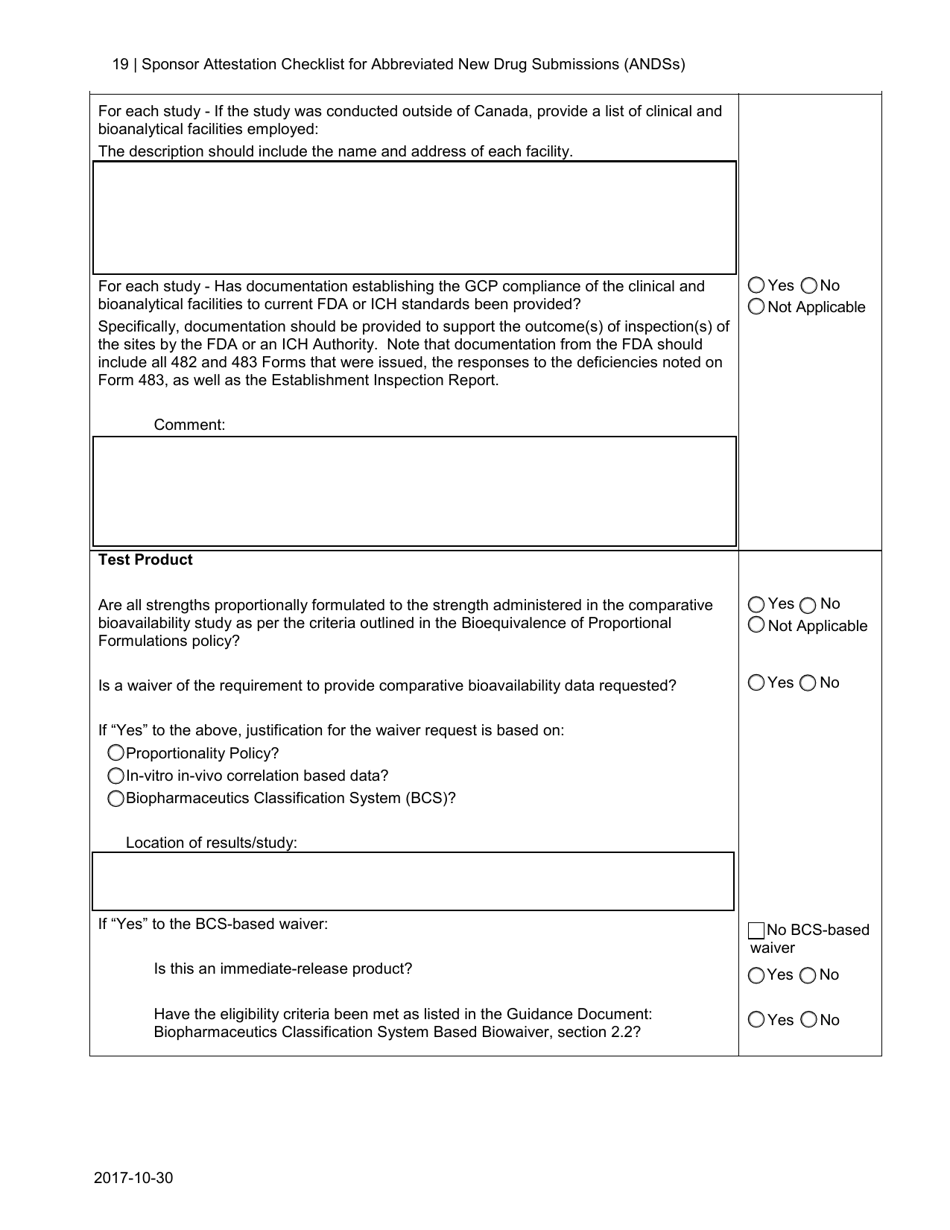
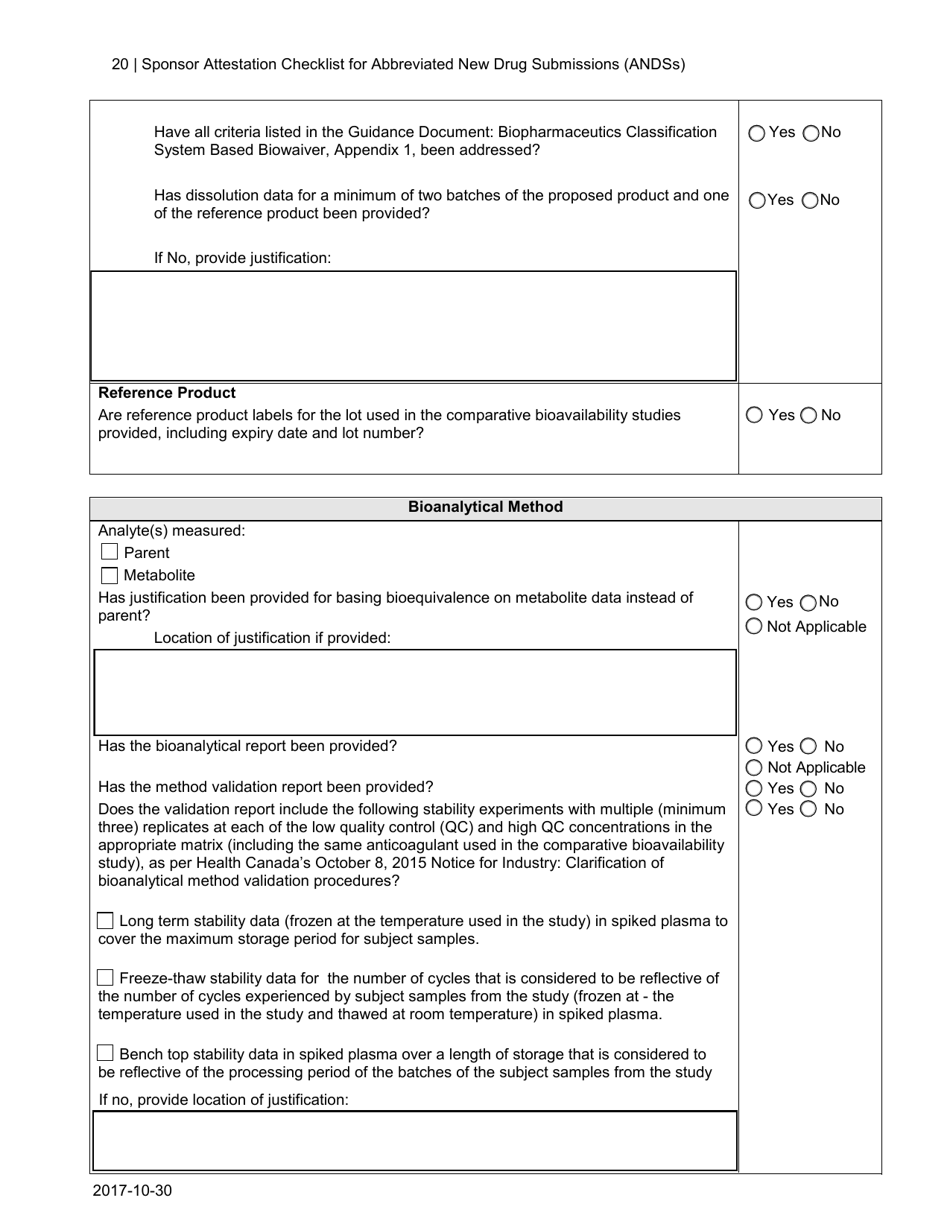
The Sponsor Attestation Checklist for Abbreviated New Drug Submissions (ANDSS) in Canada is a document that outlines the required information and attestations that a sponsor must provide when submitting an application for approval of an abbreviated new drug. It ensures that all necessary information and attestations are included in the submission.
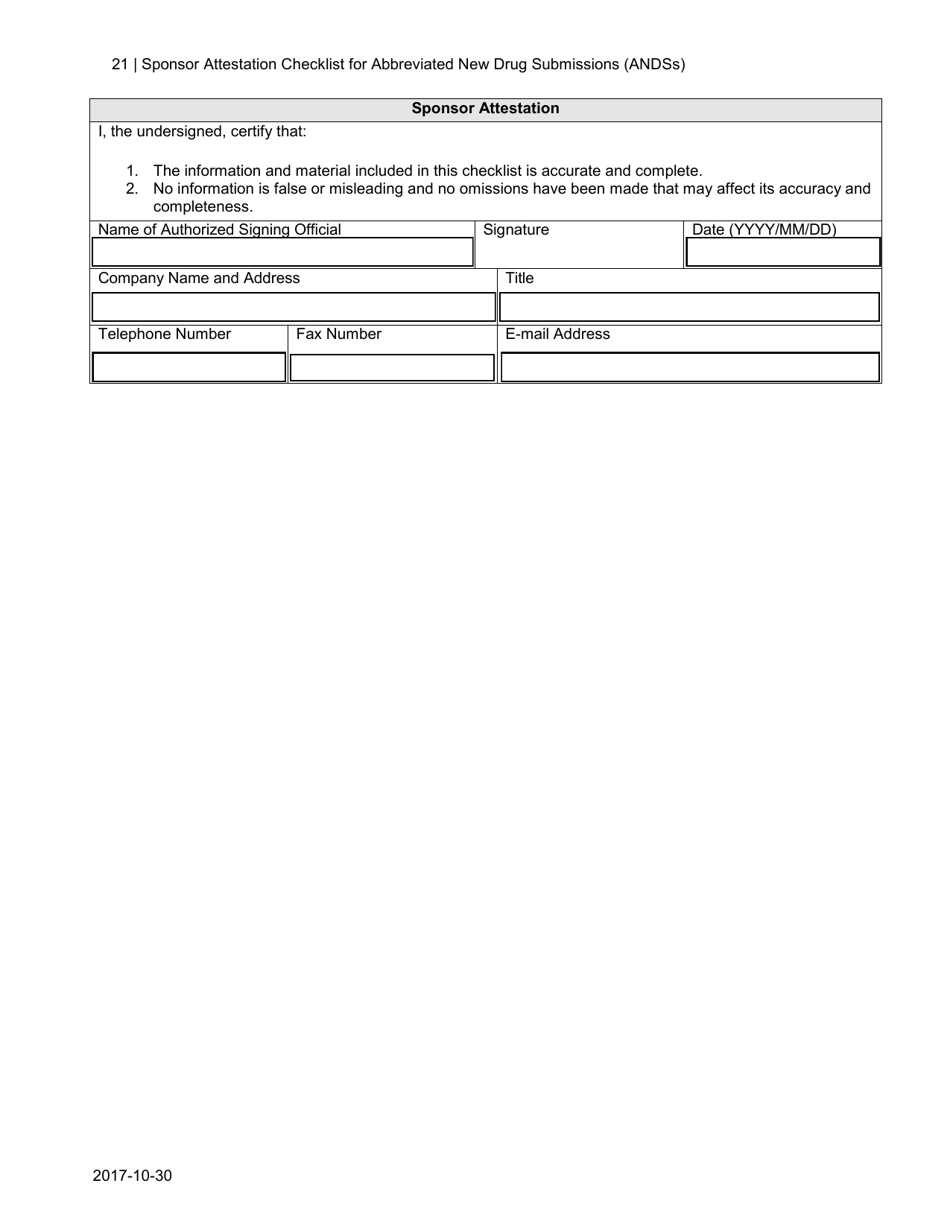
The sponsor files the Sponsor Attestation Checklist for Abbreviated New Drug Submissions (ANDSS) in Canada.
FAQ
Q: What is the Sponsor Attestation Checklist?
A: The Sponsor Attestation Checklist is a document used in Canada for Abbreviated New Drug Submissions (ANDSs).
Q: What is an Abbreviated New Drug Submission (ANDS)?
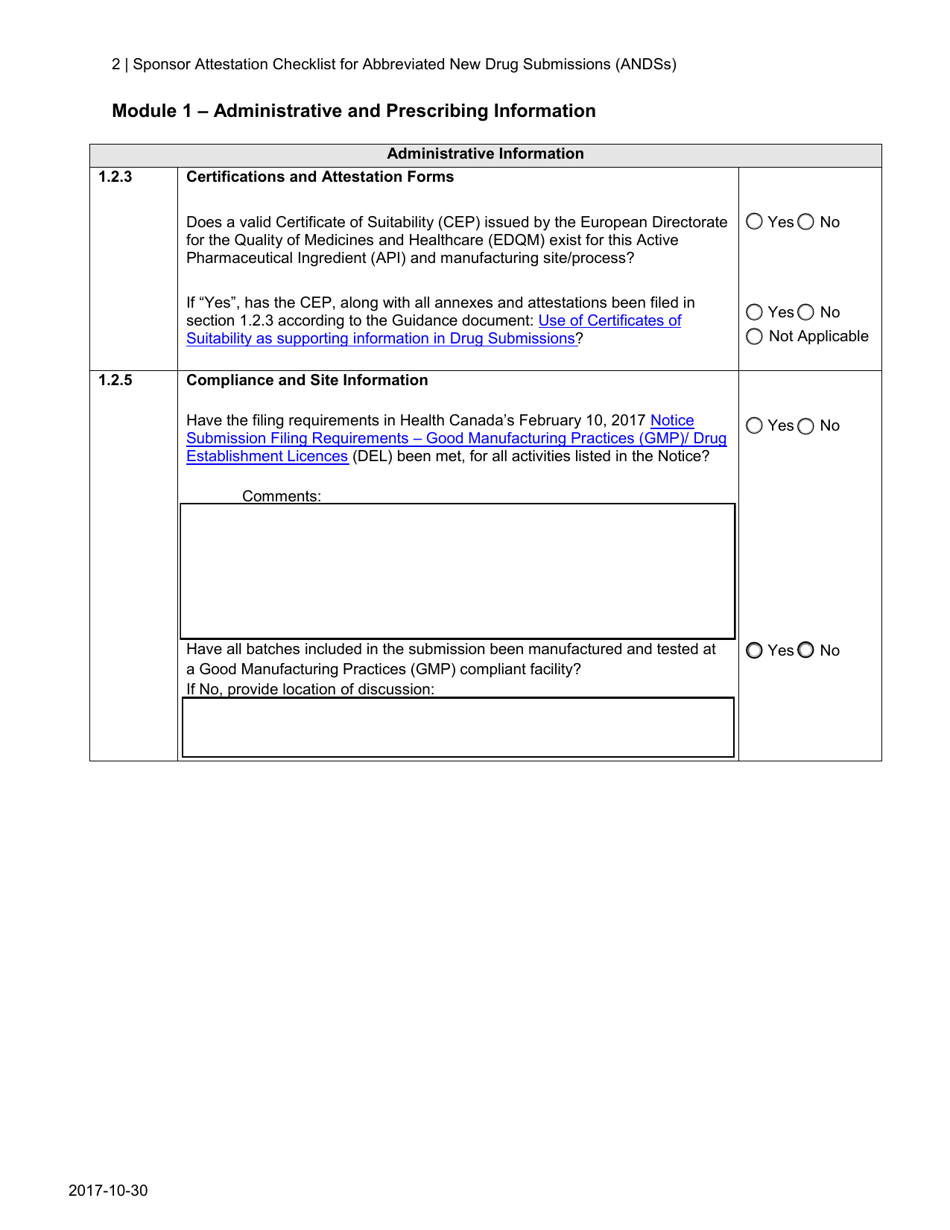
A: An Abbreviated New Drug Submission (ANDS) is a type of application submitted to Health Canada for the approval of a generic drug product.
Q: When is the Sponsor Attestation Checklist used?
A: The Sponsor Attestation Checklist is used when submitting an Abbreviated New Drug Submission (ANDS) to Health Canada.
Q: What is the purpose of the Sponsor Attestation Checklist?
A: The purpose of the Sponsor Attestation Checklist is to ensure that sponsors comply with certain requirements and provide accurate information in their Abbreviated New Drug Submission (ANDS).
Q: What information is included in the Sponsor Attestation Checklist?
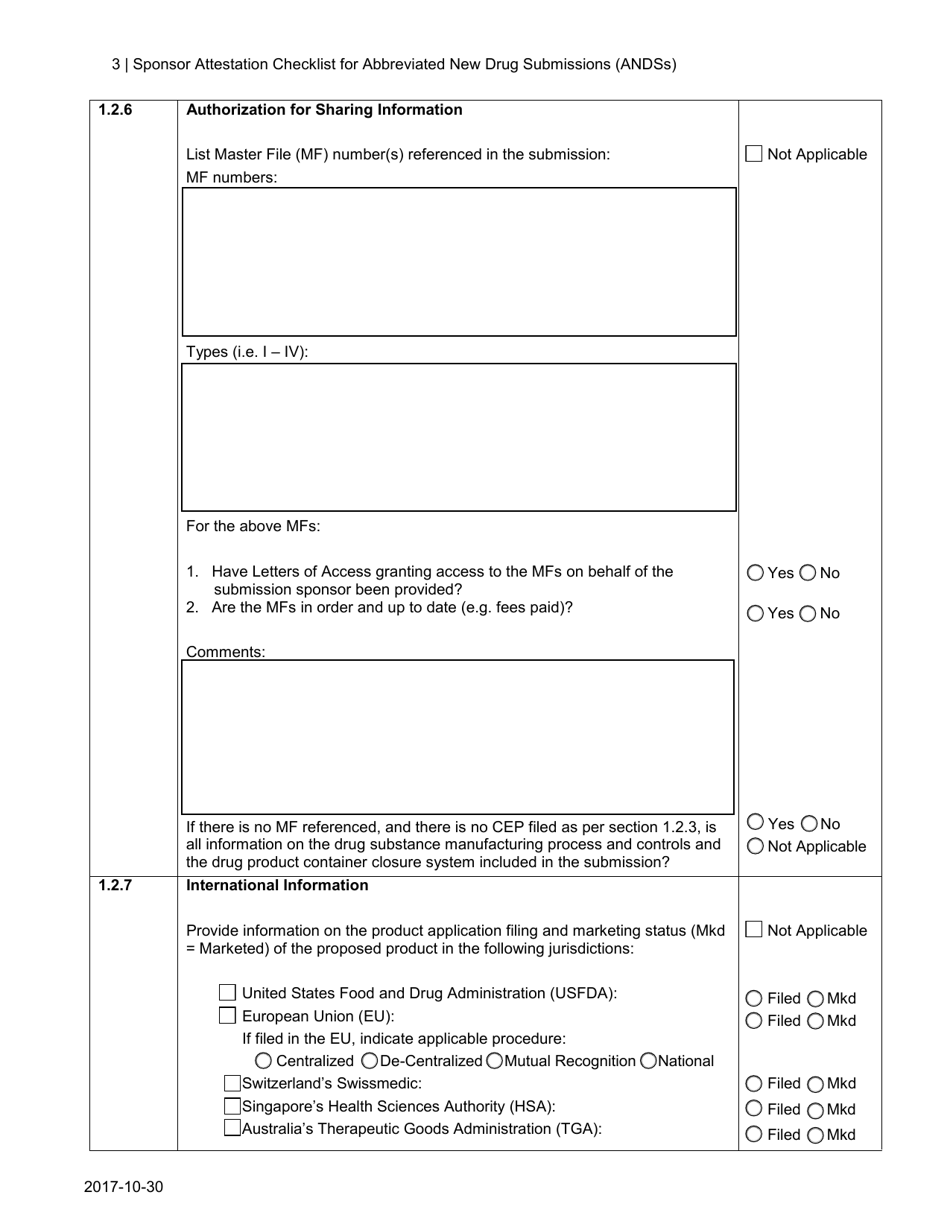
A: The Sponsor Attestation Checklist includes a list of questions and statements that sponsors must respond to or attest to regarding their ANDS.
Q: Who is responsible for completing the Sponsor Attestation Checklist?
A: The sponsor (the organization or individual submitting the ANDS) is responsible for completing the Sponsor Attestation Checklist.
Q: Can the Sponsor Attestation Checklist be submitted electronically?
A: Yes, the Sponsor Attestation Checklist can be submitted electronically along with the Abbreviated New Drug Submission (ANDS) via the Common Electronic Submission Gateway (CESG) or the Post-Approval Division (PAD) Electronic Submissions Portal.
Q: Is the Sponsor Attestation Checklist a mandatory requirement for an ANDS submission?
A: Yes, the Sponsor Attestation Checklist is a mandatory requirement for an Abbreviated New Drug Submission (ANDS) in Canada.
Q: Are there any consequences for not complying with the requirements of the Sponsor Attestation Checklist?
A: Failure to comply with the requirements of the Sponsor Attestation Checklist may result in delays or rejection of the Abbreviated New Drug Submission (ANDS).