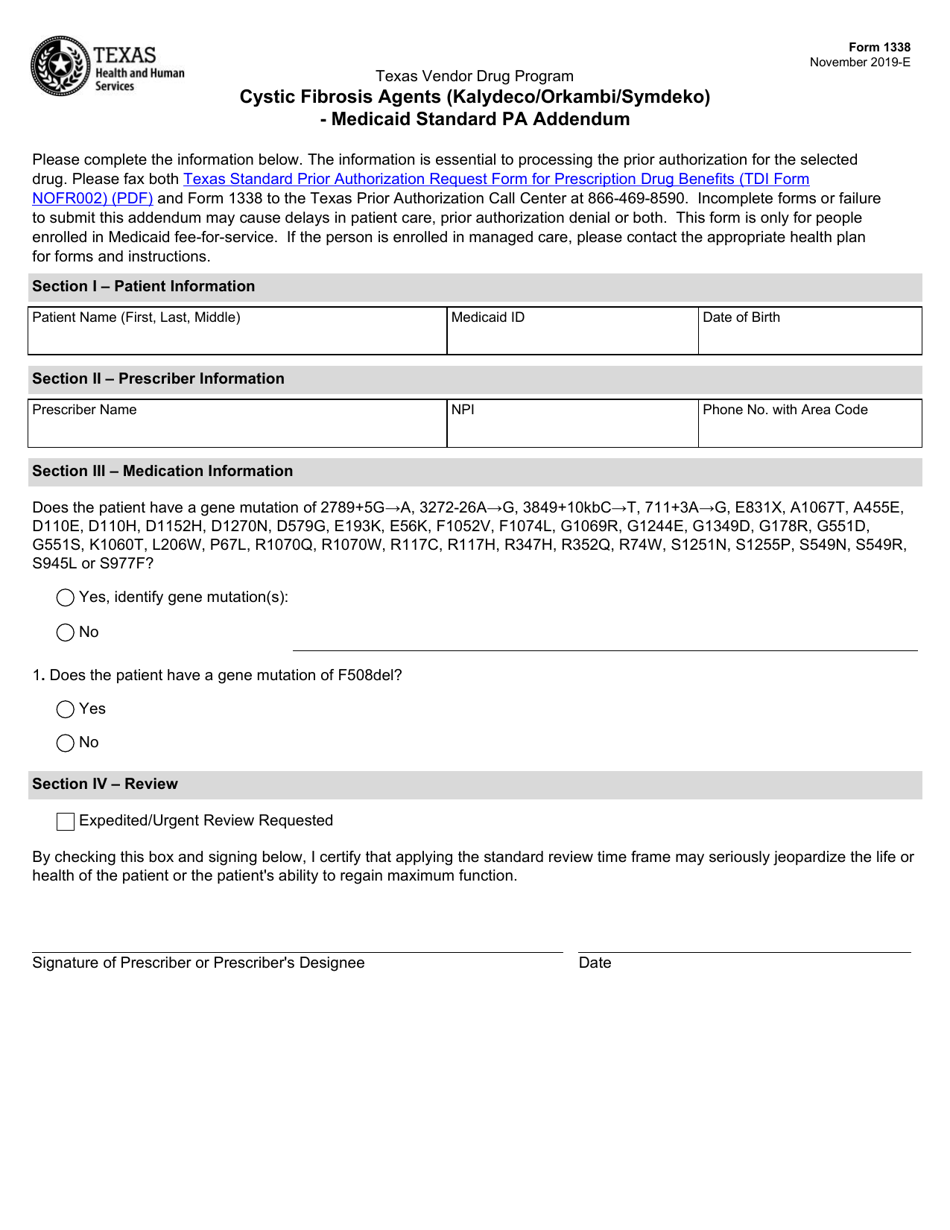
This version of the form is not currently in use and is provided for reference only. Download this version of
Form 1338
for the current year.
Form 1338 Cystic Fibrosis Agents (Kalydeco / Orkambi / Symdeko) " Medicaid Standard Pa Addendum - Texas
What Is Form 1338?
This is a legal form that was released by the Texas Health and Human Services - a government authority operating within Texas. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form 1338?
A: Form 1338 is the Medicaid Standard PA Addendum for Cystic Fibrosis Agents (Kalydeco/Orkambi/Symdeko) in Texas.
Q: What does Form 1338 cover?
A: Form 1338 covers Cystic Fibrosis Agents such as Kalydeco, Orkambi, and Symdeko.
Q: What is Medicaid Standard PA Addendum?
A: Medicaid Standard PA Addendum is a form used to request prior authorization for specific medications under the Medicaid program.
Q: What are Kalydeco, Orkambi, and Symdeko?
A: Kalydeco, Orkambi, and Symdeko are medications used to treat Cystic Fibrosis, a genetic disorder affecting the lungs and digestive system.
Q: Who is eligible for Medicaid in Texas?
A: Eligibility for Medicaid in Texas is based on income and other factors. It is typically available to low-income individuals and families.
Q: How can I get prior authorization for Cystic Fibrosis Agents?
A: You can request prior authorization for Cystic Fibrosis Agents by submitting Form 1338, the Medicaid Standard PA Addendum.
Form Details:
- Released on November 1, 2019;
- The latest edition provided by the Texas Health and Human Services;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form 1338 by clicking the link below or browse more documents and templates provided by the Texas Health and Human Services.