Post-notice of Compliance Changes: Level Iii - Canada
Health Canada files the Post-Notice of Compliance Changes for Level III in Canada.
FAQ
Q: What is a Notice of Compliance?
A: A Notice of Compliance is a document issued by Health Canada that indicates a product has met the requirements for safety, efficacy, and quality.
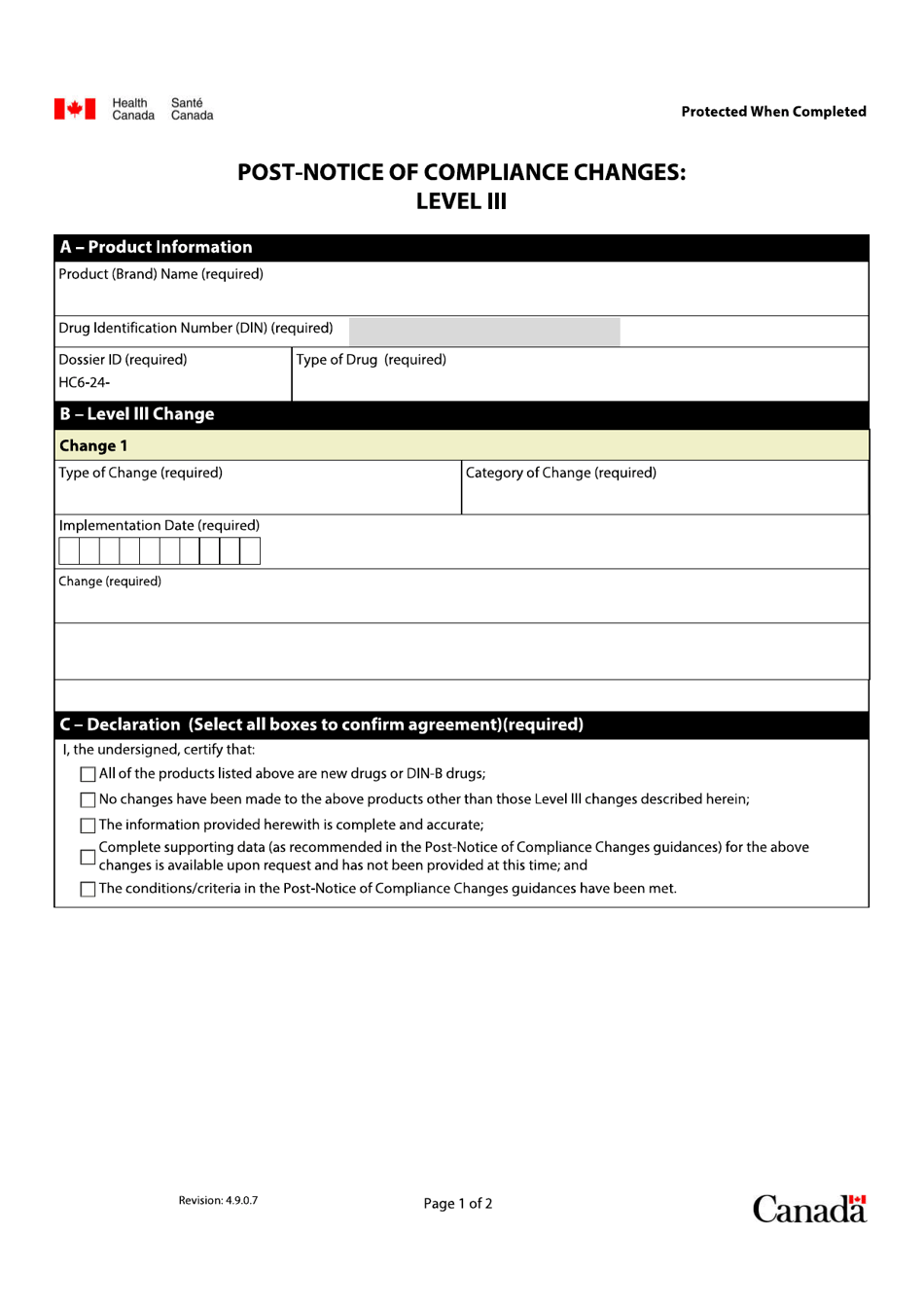
Q: What are Level III changes?
A: Level III changes refer to significant changes made to a product that may impact its safety, efficacy, or quality.
Q: Why are Level III changes important?
A: Level III changes are important because they ensure that any significant changes to a product are thoroughly reviewed by Health Canada to evaluate their impact on safety, efficacy, and quality.
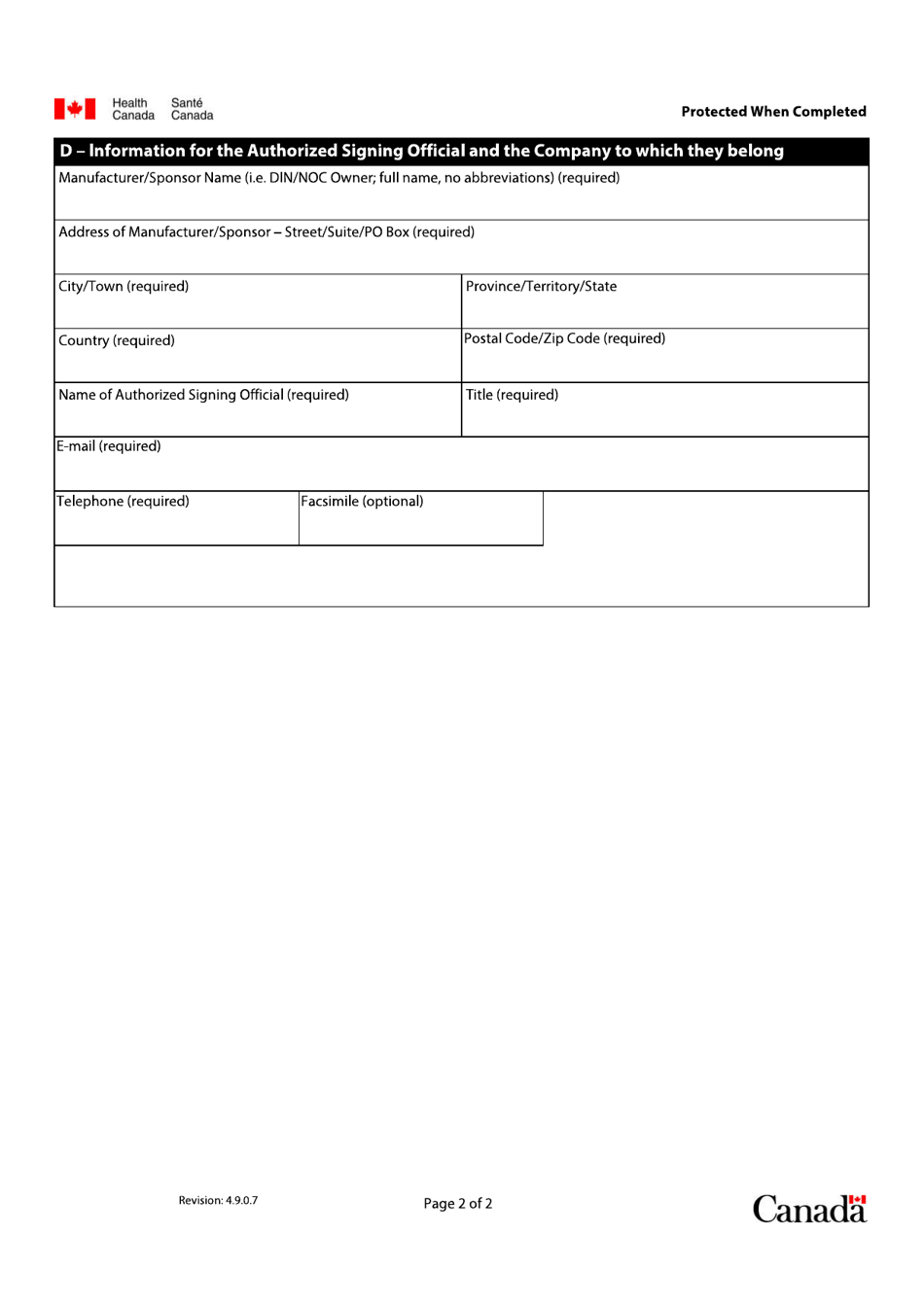
Q: What is the purpose of posting a Notice of Compliance Changes for Level III changes?
A: The purpose of posting a Notice of Compliance Changes for Level III changes is to inform the public and healthcare professionals about the changes made to a product and any associated risks or potential impacts on its use.