This version of the form is not currently in use and is provided for reference only. Download this version of
the document
for the current year.
Labels and Packages Certification Form for Prescription Products - Canada
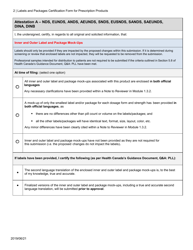
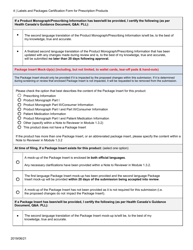
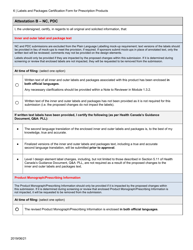
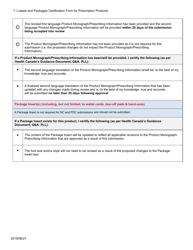
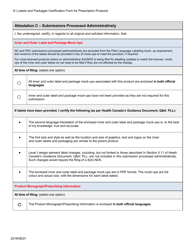
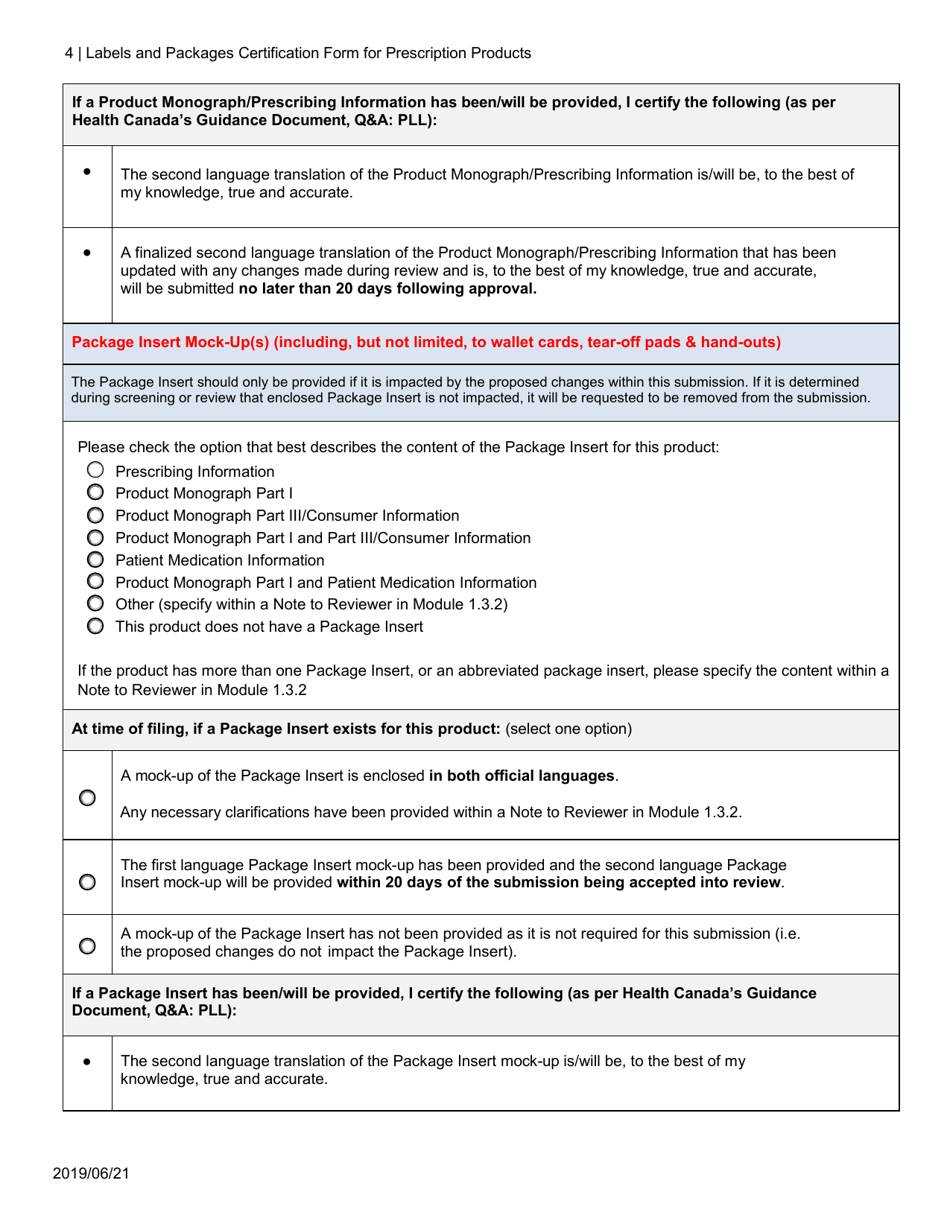
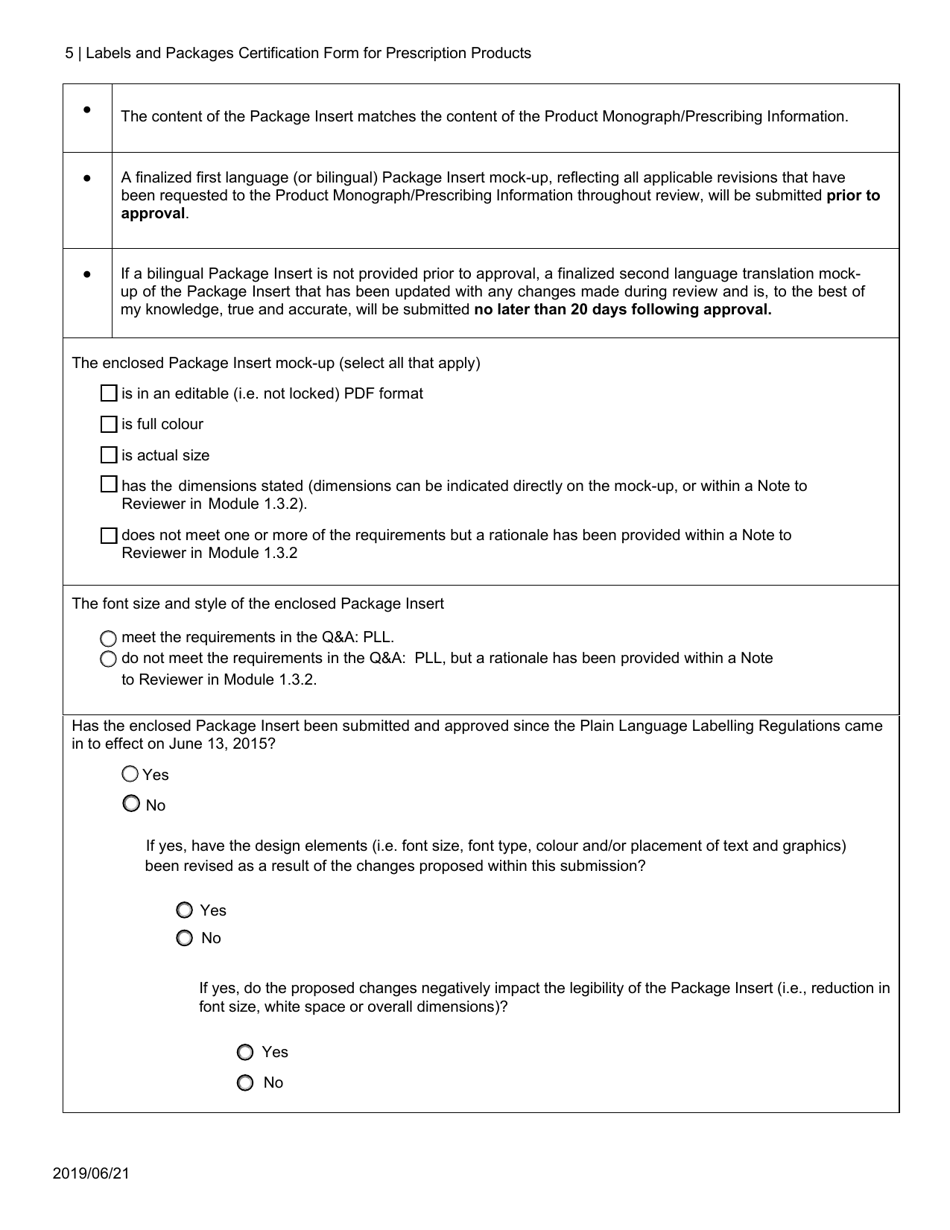
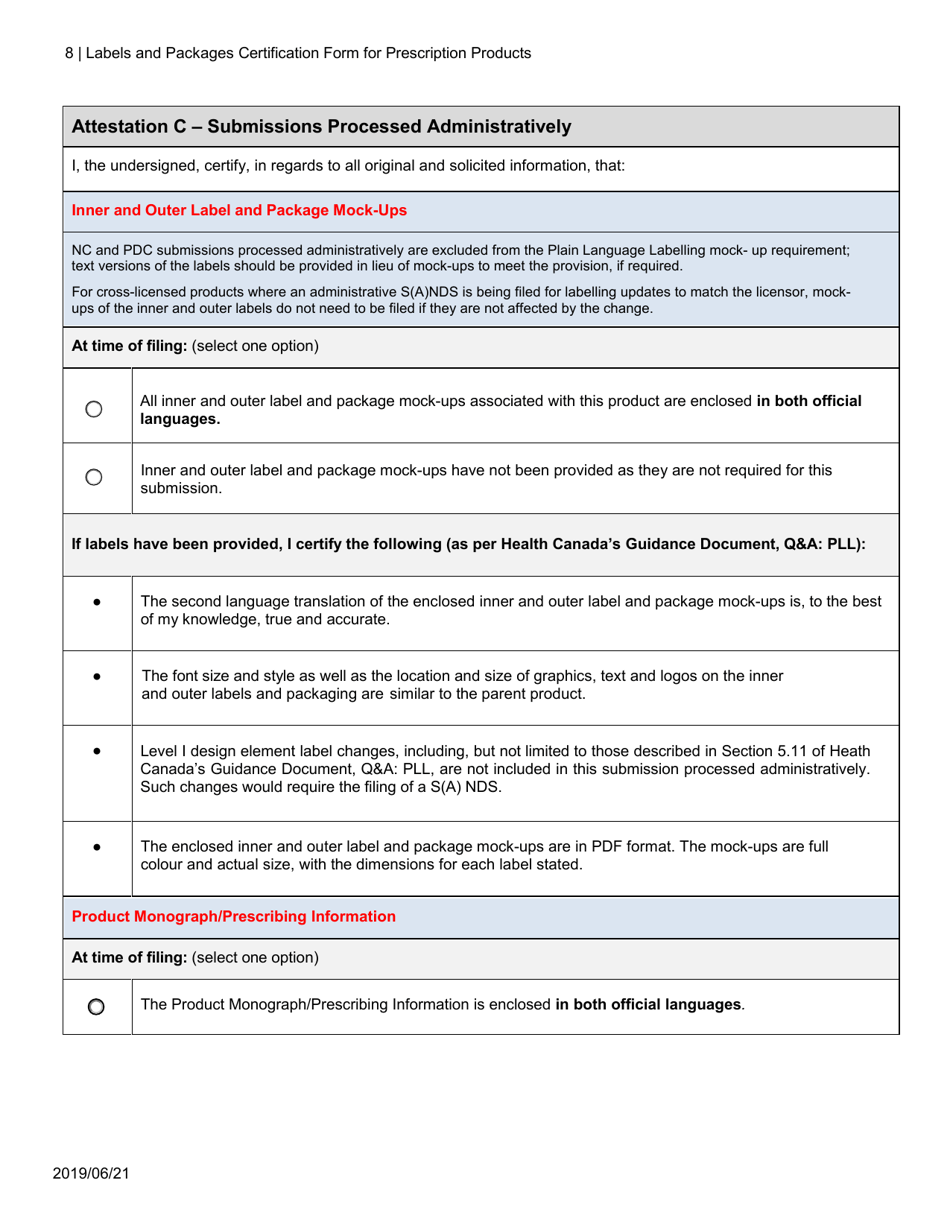
The Labels and Packages Certification Form for Prescription Products in Canada is used to certify that the labels and packages of prescription drugs meet the regulatory requirements set by Health Canada. It ensures that the information provided on the labels and packages of prescription products is accurate, clear, and easily understood by consumers.
In Canada, the manufacturer or sponsor of the prescription product is responsible for filing the Labels and Packages Certification Form.
FAQ
Q: What is a Labels and Packages Certification Form?
A: The Labels and Packages Certification Form is a document used in Canada to certify that the labels and packages of prescription products comply with the regulations.
Q: Why is the Labels and Packages Certification Form important?
A: The form is important because it ensures that prescription products have accurate and clear labels and packages, which is crucial for patient safety and information.
Q: Who needs to complete the Labels and Packages Certification Form?
A: Manufacturers or importers of prescription products in Canada need to complete the form.
Q: What information is included in the form?
A: The form typically includes details about the product, its intended use, and the certification statement confirming compliance with regulations.
Q: Are there any fees associated with submitting the form?
A: Yes, there are fees associated with submitting the Labels and Packages Certification Form. The specific fees can vary and should be checked with the regulatory authority.
Q: Do all prescription products require this form?
A: Yes, all prescription products in Canada require the Labels and Packages Certification Form to ensure compliance with regulations.
Q: Can the form be submitted electronically?
A: Yes, in most cases, the Labels and Packages Certification Form can be submitted electronically.
Q: What happens after submitting the Labels and Packages Certification Form?
A: After submitting the form, it will be reviewed by the regulatory authority. If the product is found to comply with regulations, it will be authorized for sale in Canada.
Q: How long does it take to receive authorization after submitting the form?
A: The timeframe for receiving authorization can vary, but it typically takes several weeks to months for the review process to be completed.