This version of the form is not currently in use and is provided for reference only. Download this version of
Form FA-62
for the current year.
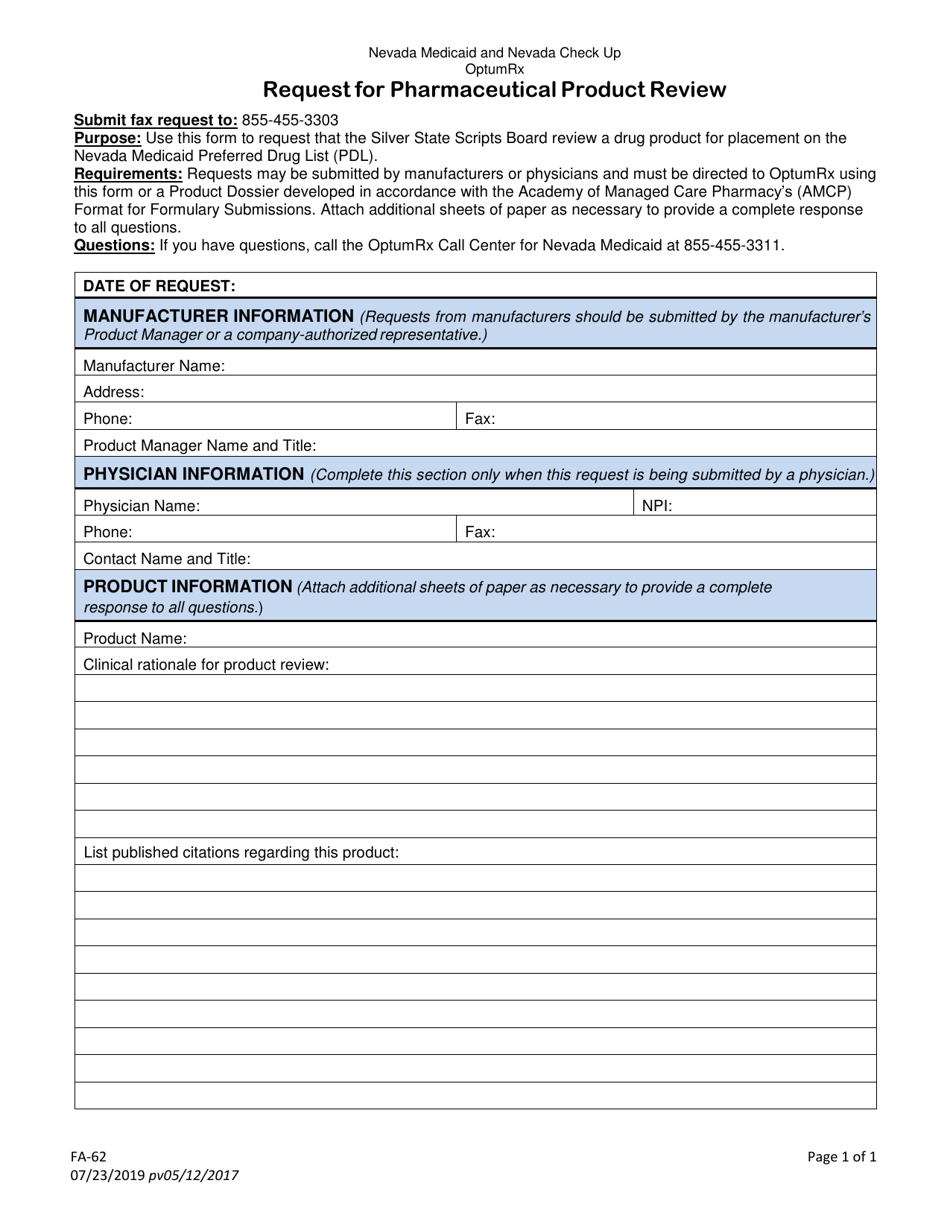
Form FA-62 Request for Pharmaceutical Product Review - Nevada
What Is Form FA-62?
This is a legal form that was released by the Nevada Department of Health and Human Services - a government authority operating within Nevada. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form FA-62?
A: Form FA-62 is the Request for Pharmaceutical Product Review in Nevada.
Q: Who can submit Form FA-62?
A: Pharmaceutical companies can submit Form FA-62 for the review of their products in Nevada.
Q: What is the purpose of Form FA-62?
A: The purpose of Form FA-62 is to request a review of a pharmaceutical product in Nevada.
Q: How can I obtain Form FA-62?
A: Form FA-62 can be obtained from the Nevada Department of Health and Human Services.
Q: What information is required on Form FA-62?
A: Form FA-62 requires information such as the product name, manufacturer information, and the purpose of the review.
Q: How long does the review process take?
A: The review process time can vary. It is best to check with the Nevada Department of Health and Human Services for the current processing times.
Q: What happens after the review?
A: After the review, the Nevada Department of Health and Human Services will provide a decision on the product.
Q: Can I appeal the decision?
A: Yes, there is an appeals process available if you disagree with the decision made by the Nevada Department of Health and Human Services.
Q: Are there any additional requirements after the review?
A: Yes, there may be additional requirements or conditions imposed on the product after the review.
Q: Can I submit Form FA-62 by mail?
A: Yes, Form FA-62 can be submitted by mail to the address provided on the form.
Form Details:
- Released on July 23, 2019;
- The latest edition provided by the Nevada Department of Health and Human Services;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form FA-62 by clicking the link below or browse more documents and templates provided by the Nevada Department of Health and Human Services.