Form DBPR-DDC-236 Application for Permit as a Nonresident Prescription Drug Manufacturer - Virtual - Florida
What Is Form DBPR-DDC-236?
This is a legal form that was released by the Florida Department of Business & Professional Regulation - a government authority operating within Florida. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is DBPR-DDC-236?
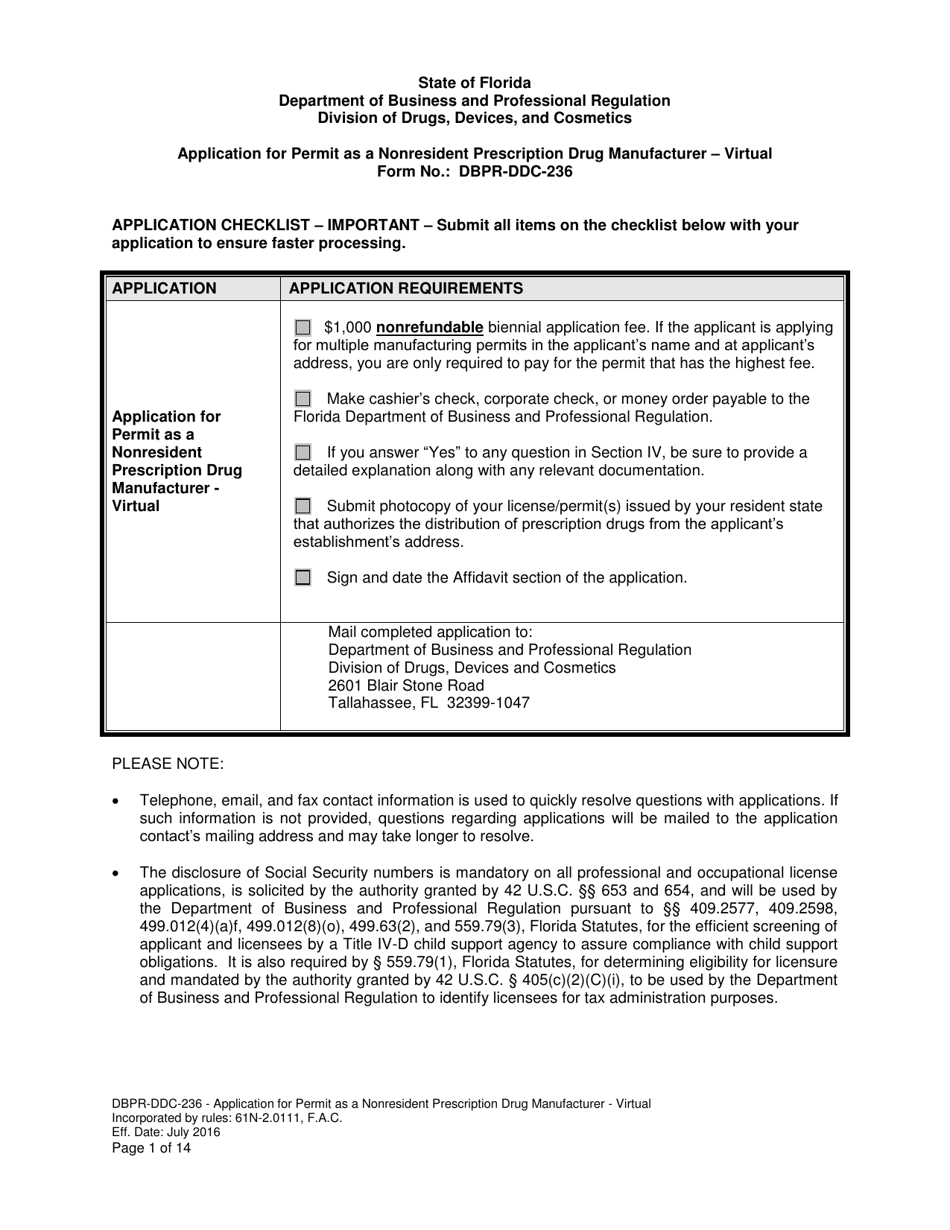
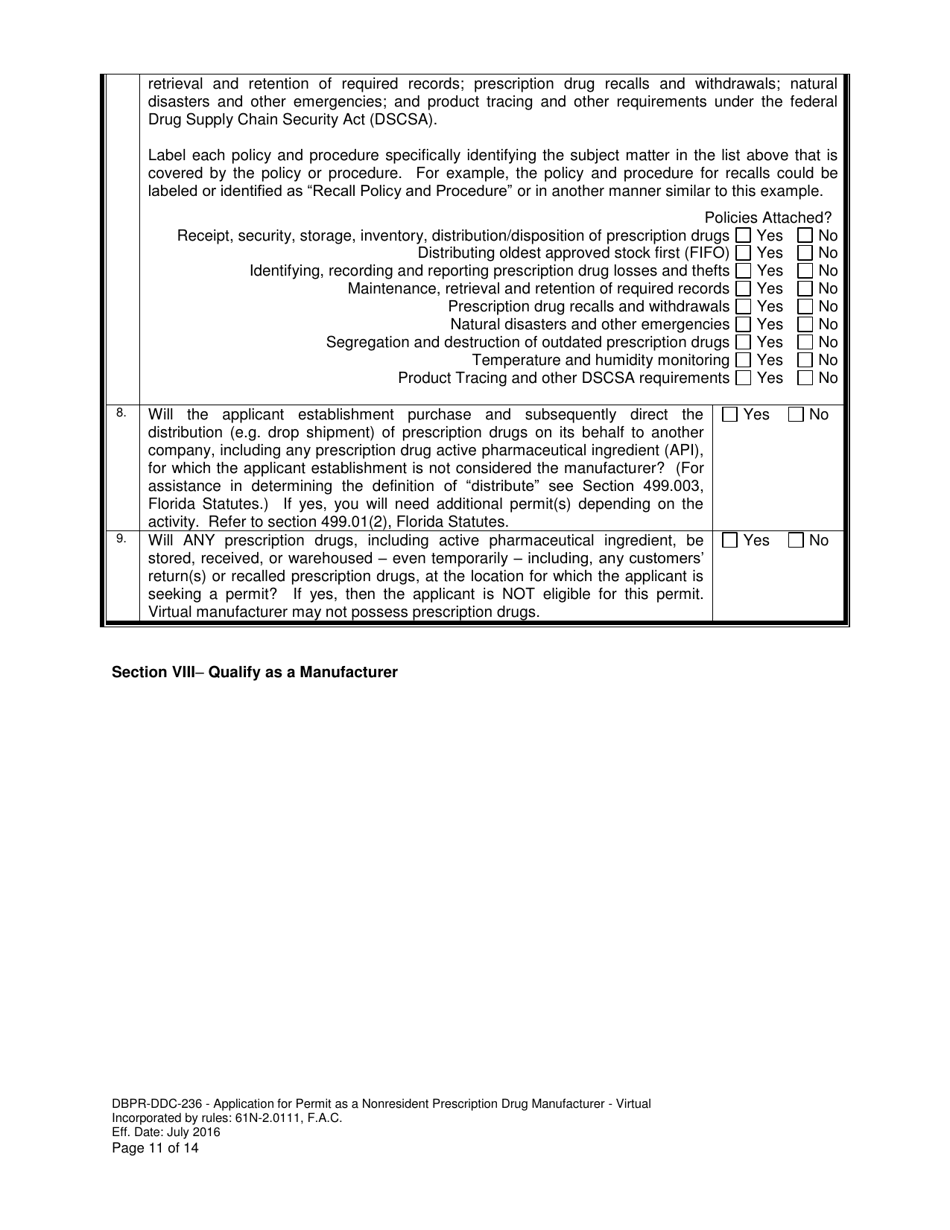
A: DBPR-DDC-236 is an application for a Permit as a Nonresident Prescription Drug Manufacturer in Florida.
Q: Who can use DBPR-DDC-236?
A: Nonresident Prescription Drug Manufacturers who wish to obtain a permit in Florida can use DBPR-DDC-236.
Q: What is a Nonresident Prescription Drug Manufacturer?
A: A Nonresident Prescription Drug Manufacturer is a company that produces prescription drugs outside of Florida and wants to sell them in the state.
Q: Why do I need a permit as a Nonresident Prescription Drug Manufacturer in Florida?
A: You need a permit to ensure compliance with the state's regulations and to legally sell prescription drugs in Florida.
Q: What information do I need to provide in DBPR-DDC-236?
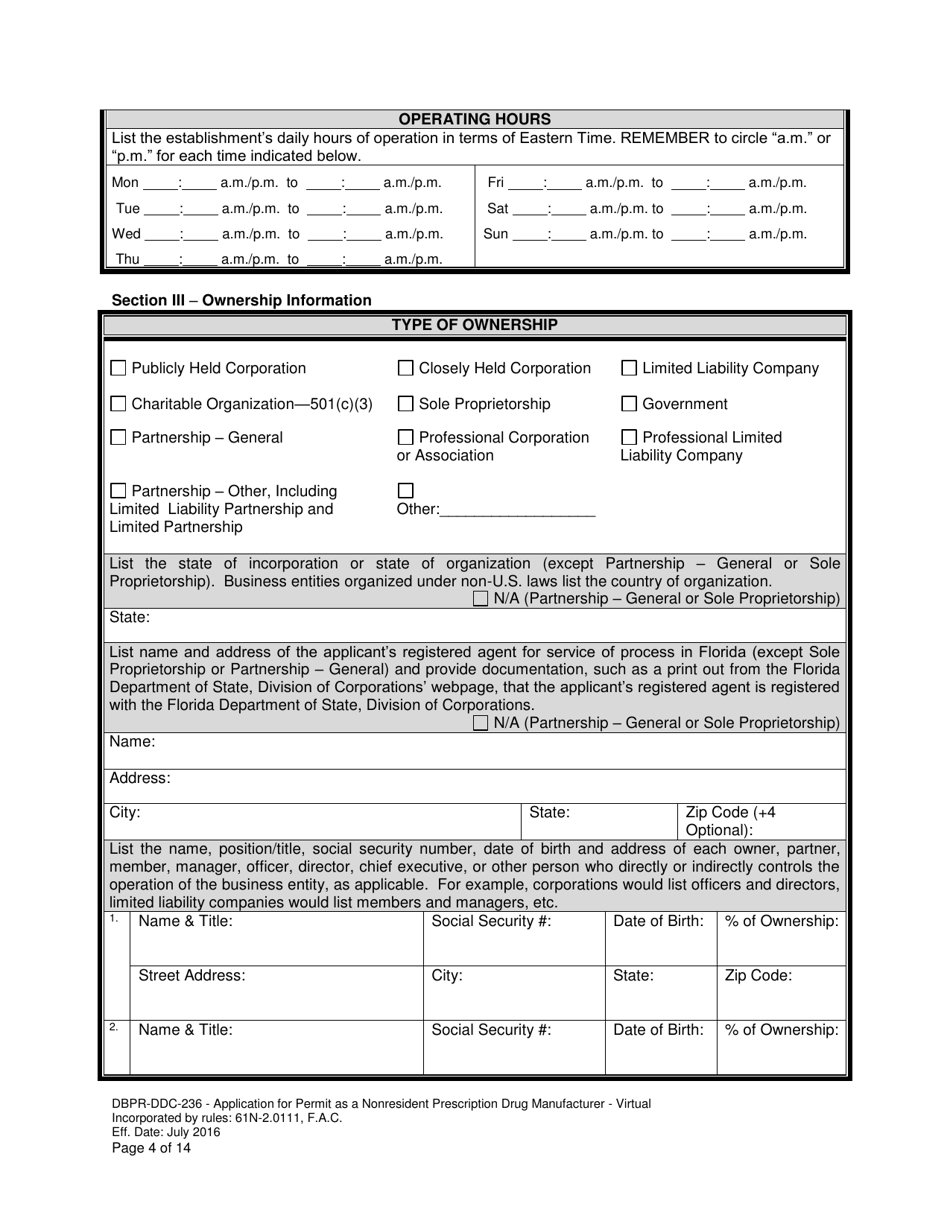
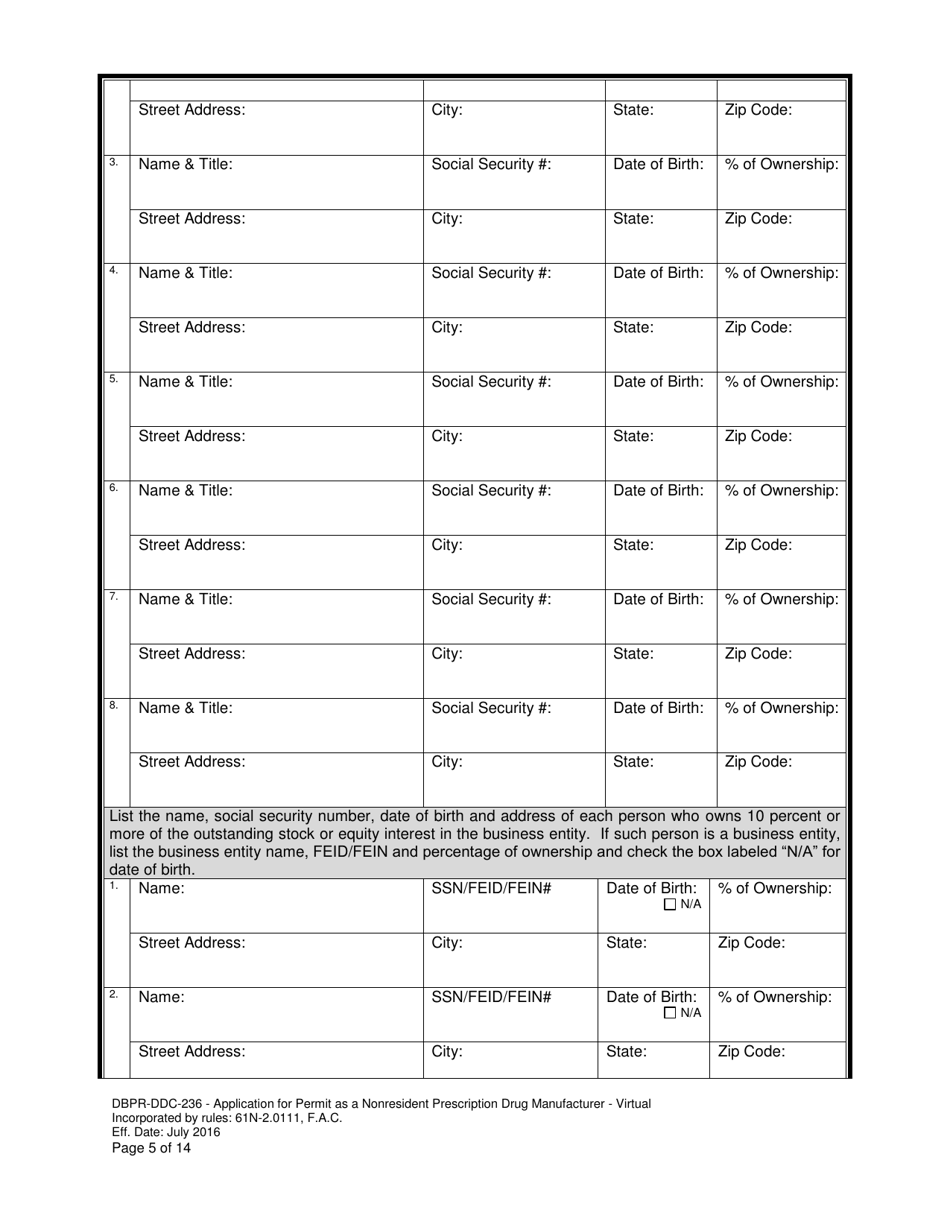
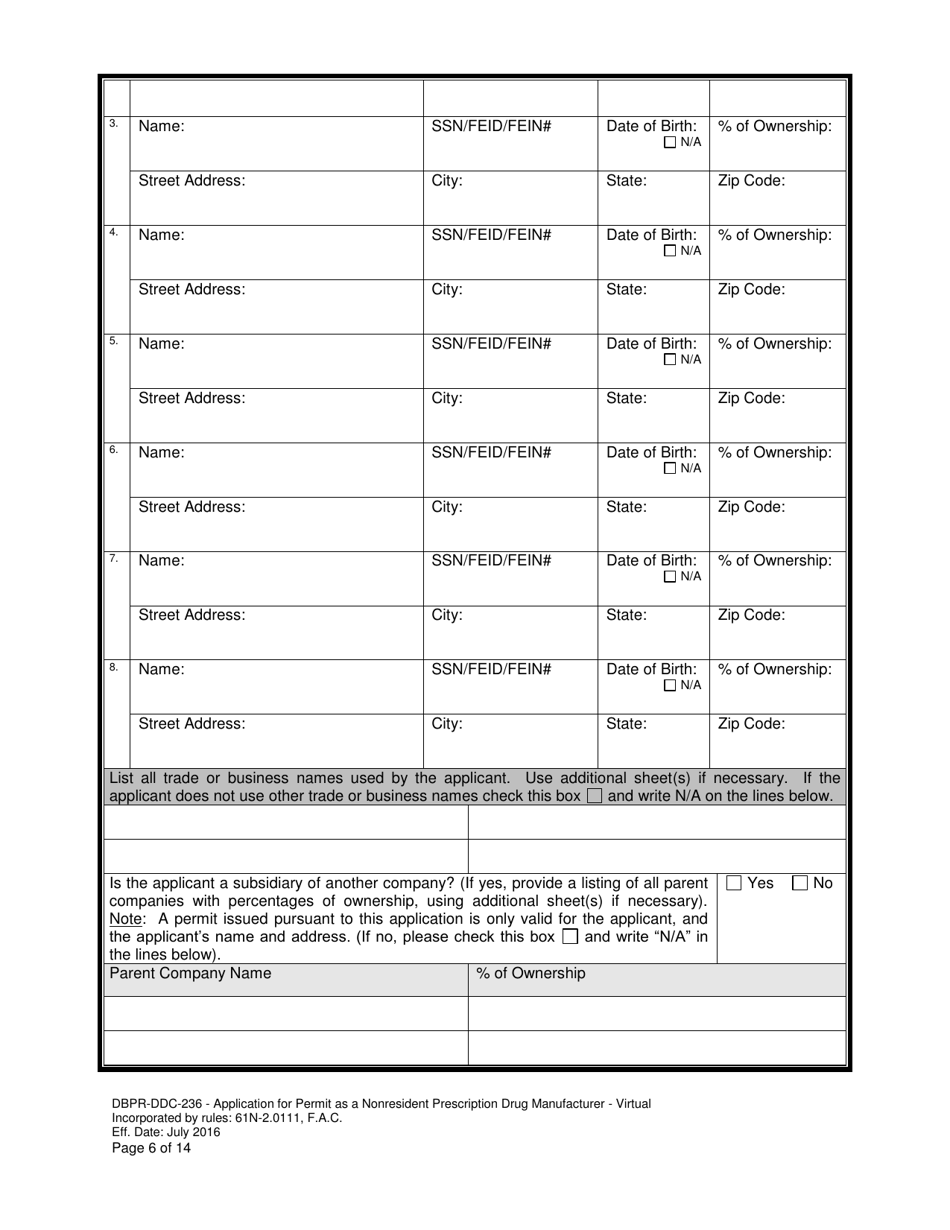
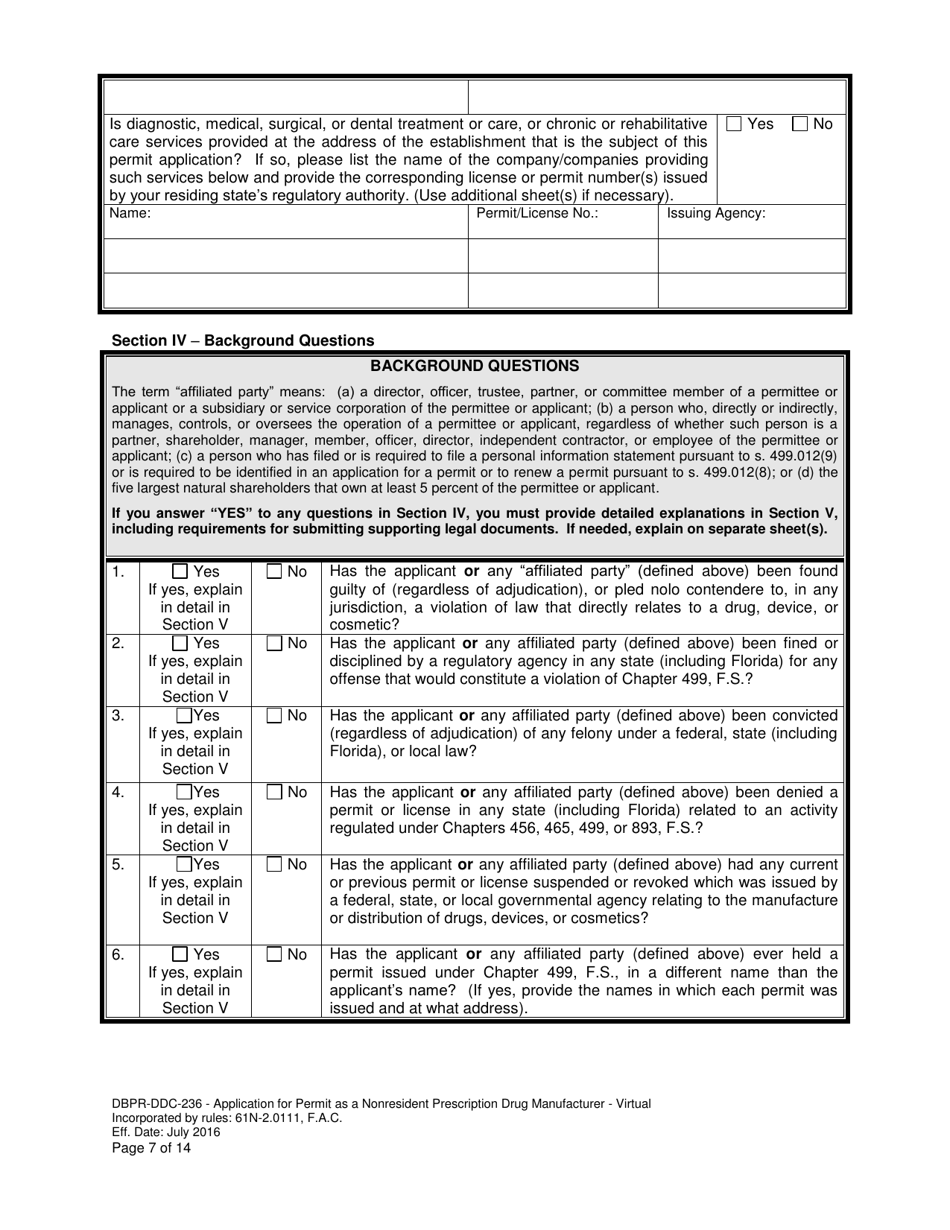
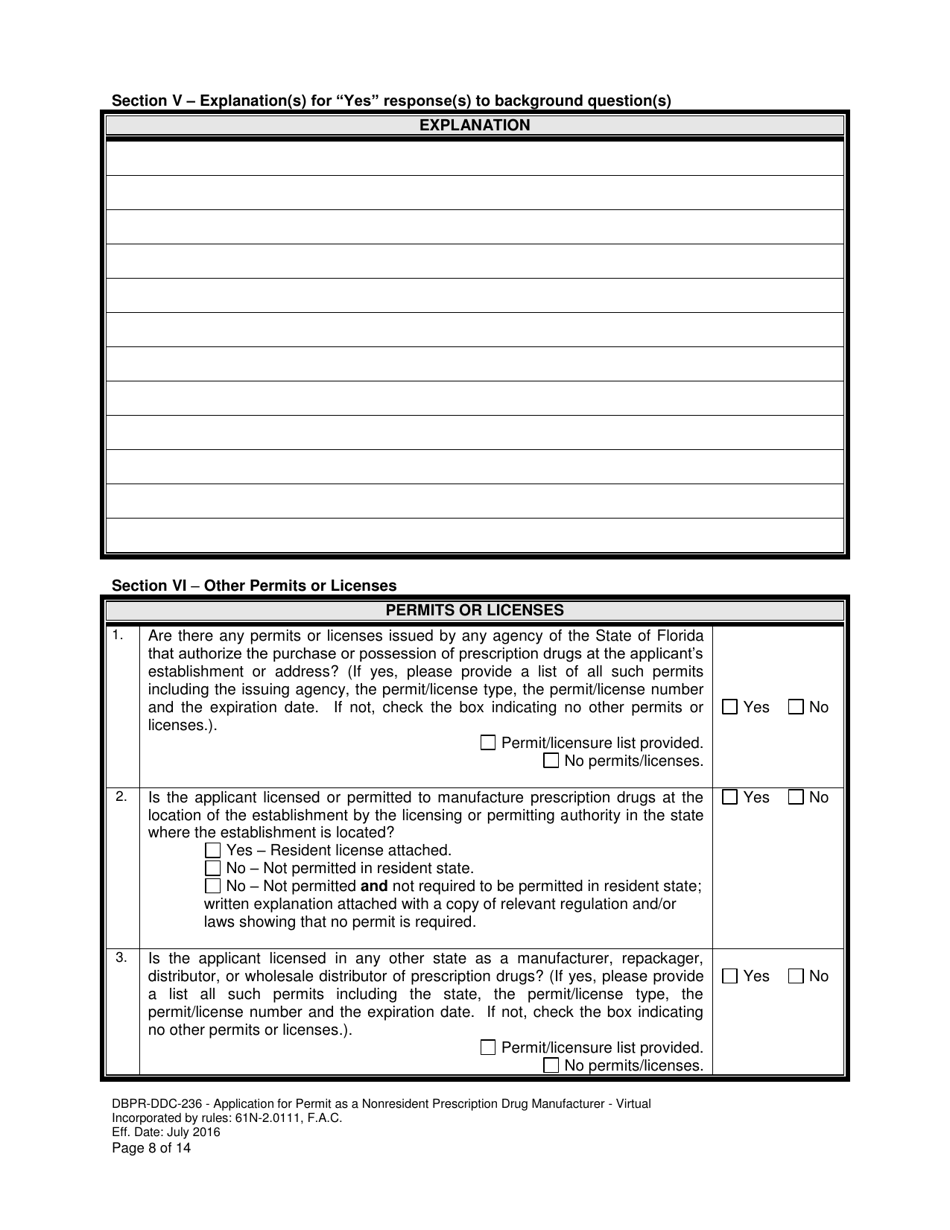
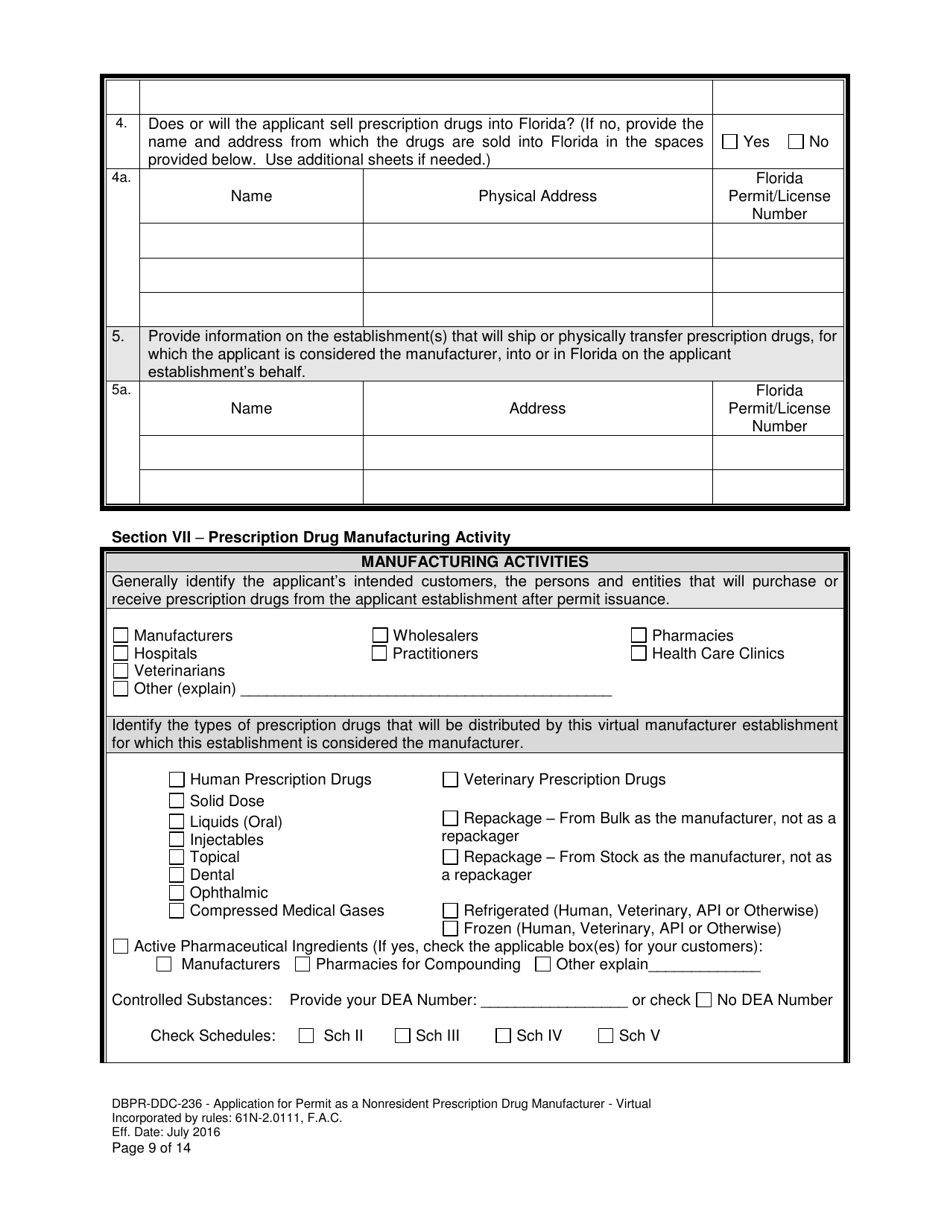
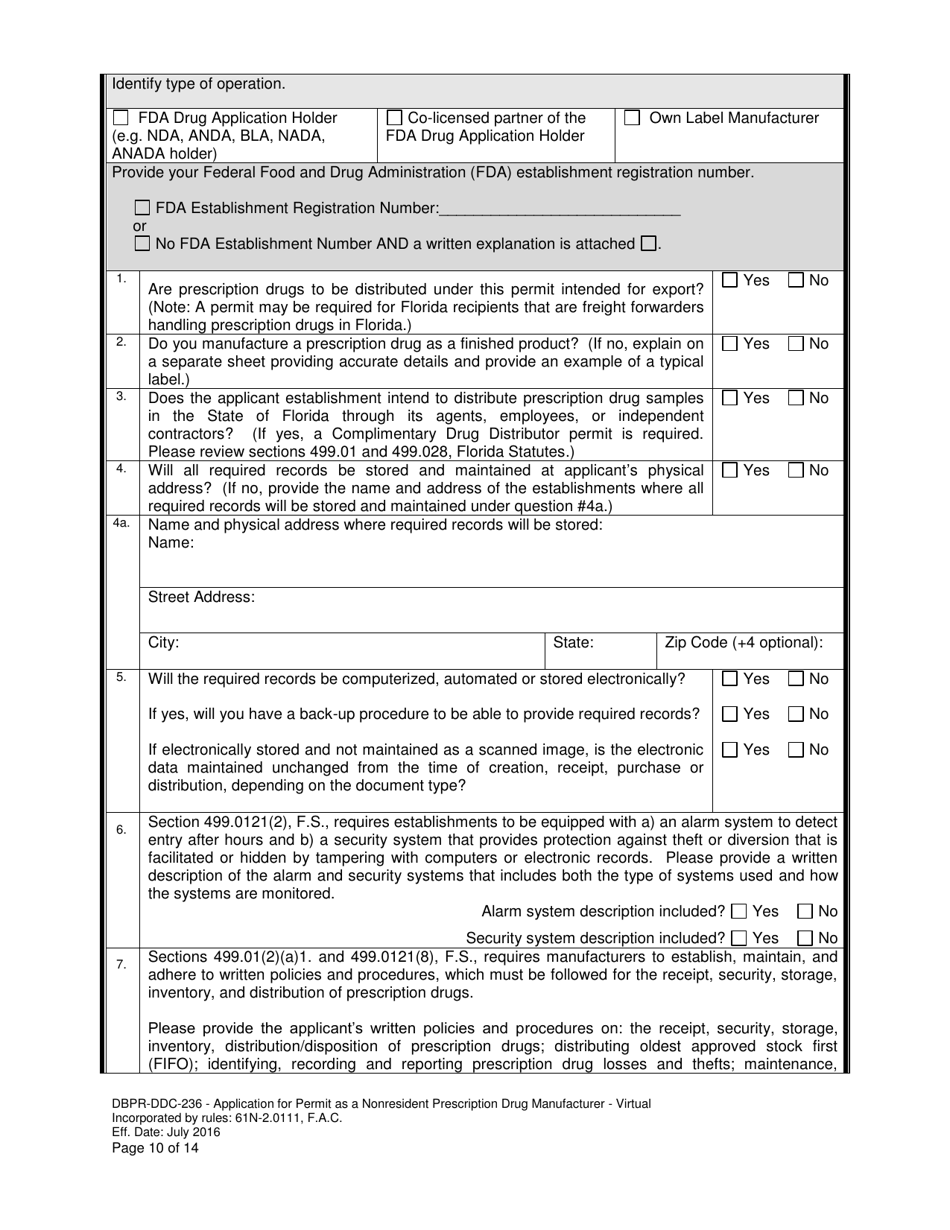
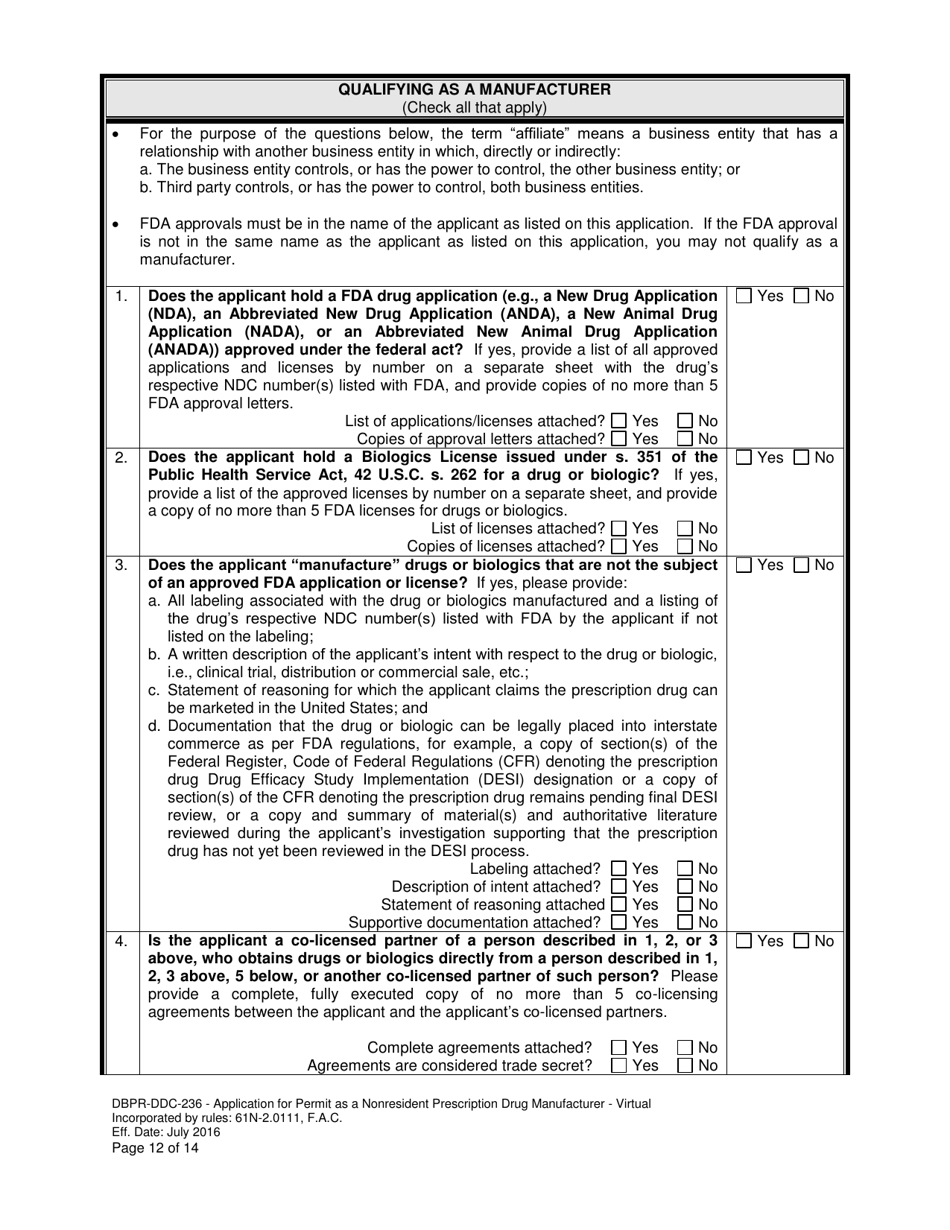
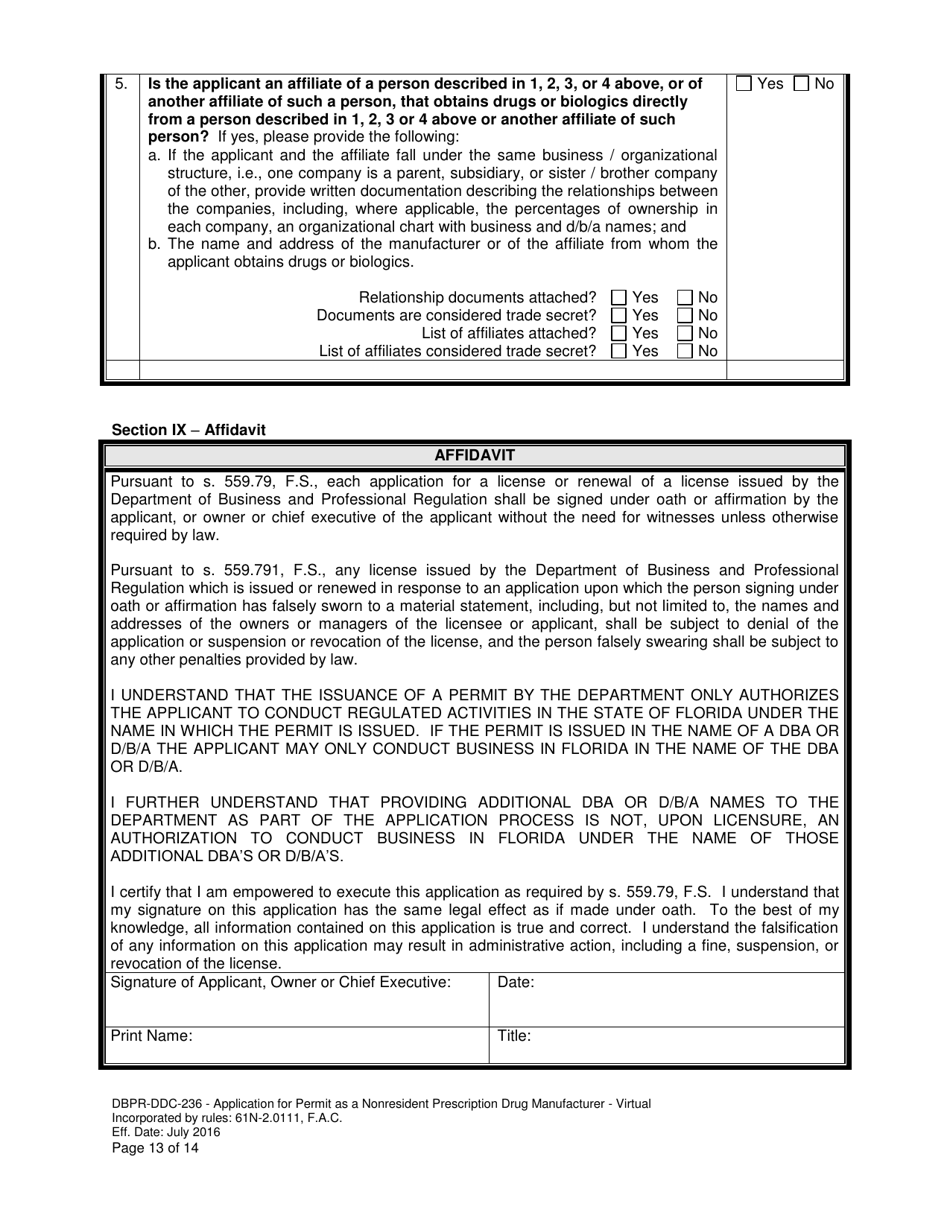
A: DBPR-DDC-236 requires various information such as company details, manufacturing processes, compliance records, and more. The specific requirements can be found on the application form.
Q: How long does it take to process the DBPR-DDC-236 application?
A: The processing time for the DBPR-DDC-236 application may vary. It is recommended to check with the DBPR for the current processing times.
Form Details:
- Released on July 1, 2016;
- The latest edition provided by the Florida Department of Business & Professional Regulation;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of Form DBPR-DDC-236 by clicking the link below or browse more documents and templates provided by the Florida Department of Business & Professional Regulation.