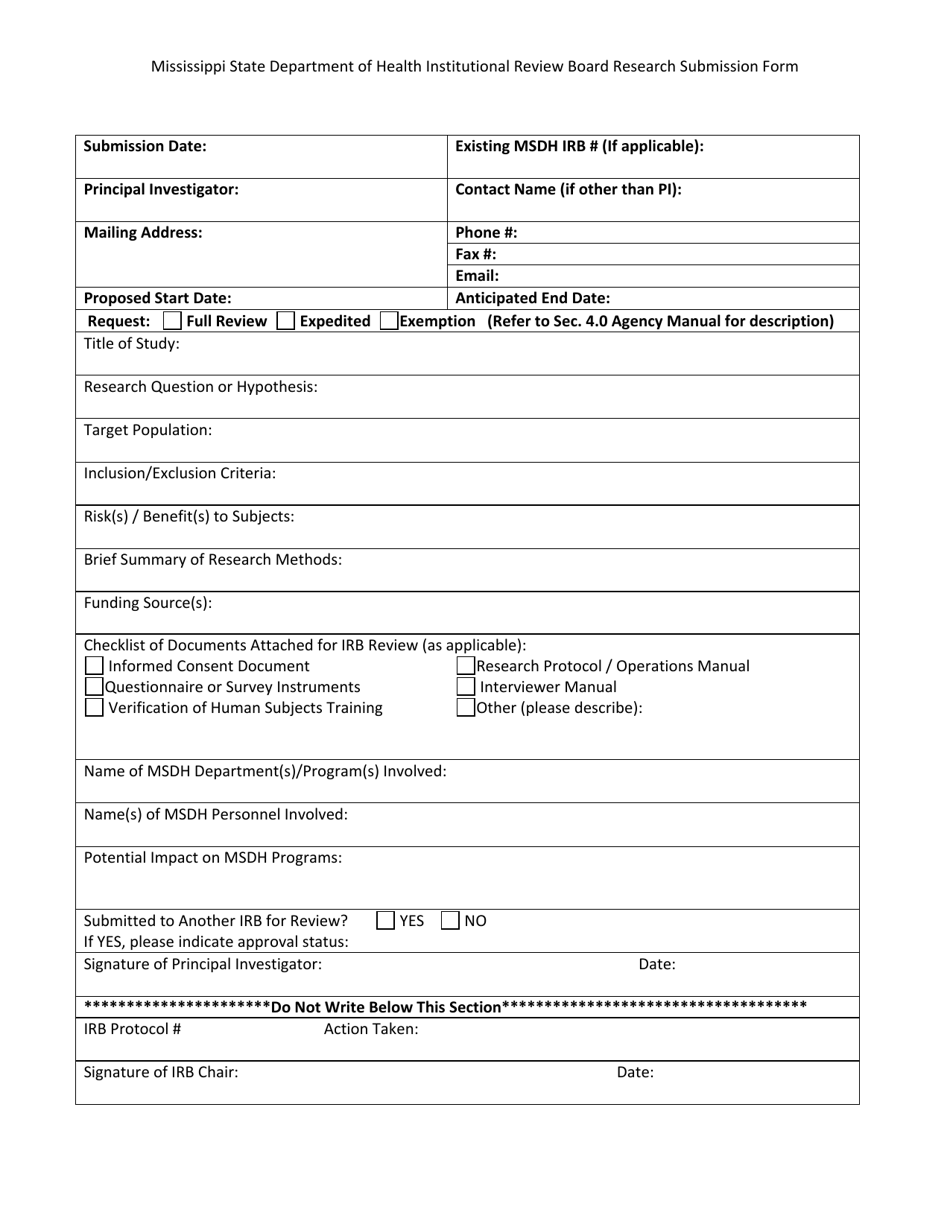
Institutional Review Board Research Submission Form - Mississippi
Institutional Review Board Research Submission Form is a legal document that was released by the Mississippi Department of Health - a government authority operating within Mississippi.
FAQ
Q: What is the Institutional Review Board (IRB)?
A: The Institutional Review Board (IRB) is a committee that reviews and approves research involving human subjects.
Q: Why is an IRB review necessary for research involving human subjects?
A: An IRB review is necessary to ensure that research involving human subjects is conducted ethically, with respect for the rights and welfare of the participants.
Q: What is the purpose of the Institutional Review Board Research Submission Form?
A: The purpose of the Institutional Review Board Research Submission Form is to provide the necessary information about a research study for the IRB to review and determine if it meets ethical standards.
Q: Who needs to complete the Institutional Review Board Research Submission Form?
A: Researchers who are planning to conduct research involving human subjects need to complete the Institutional Review Board Research Submission Form.
Q: What information is typically required in the Institutional Review Board Research Submission Form?
A: The Institutional Review Board Research Submission Form typically requires information about the study objectives, methodology, risks and benefits to participants, and plans for obtaining informed consent.
Q: Are there any deadlines for submitting the Institutional Review Board Research Submission Form?
A: Deadlines for submitting the Institutional Review Board Research Submission Form may vary depending on the institution and the specific research study. It is best to check with the IRB or research office for the deadlines.
Q: What happens after submitting the Institutional Review Board Research Submission Form?
A: After submitting the Institutional Review Board Research Submission Form, the IRB will review the research study to determine if it meets ethical standards. The researcher may be required to make revisions or provide additional information.
Q: How long does the review process by the IRB take?
A: The length of the review process by the IRB can vary depending on the complexity of the research study and the workload of the IRB. It is best to check with the IRB or research office for an estimated timeline.
Q: Can research involving human subjects proceed without IRB approval?
A: No, research involving human subjects should not proceed without IRB approval. It is important to obtain the necessary ethical approval to ensure the protection and well-being of the participants.
Form Details:
- The latest edition currently provided by the Mississippi Department of Health;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of the form by clicking the link below or browse more documents and templates provided by the Mississippi Department of Health.