Quick Reference Guide - Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations
Quick Reference Guide - Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations is a 2-page legal document that was released by the U.S. Department of Health and Human Services - Centers for Disease Control and Prevention on June 27, 2014 and used nation-wide.
FAQ
Q: What is the purpose of laboratory testing for the diagnosis of HIV infection?
A: Laboratory testing is performed to confirm the presence of HIV infection in an individual.
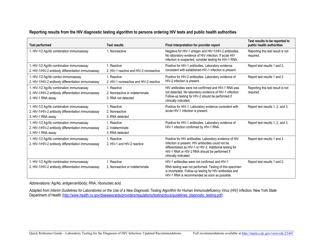
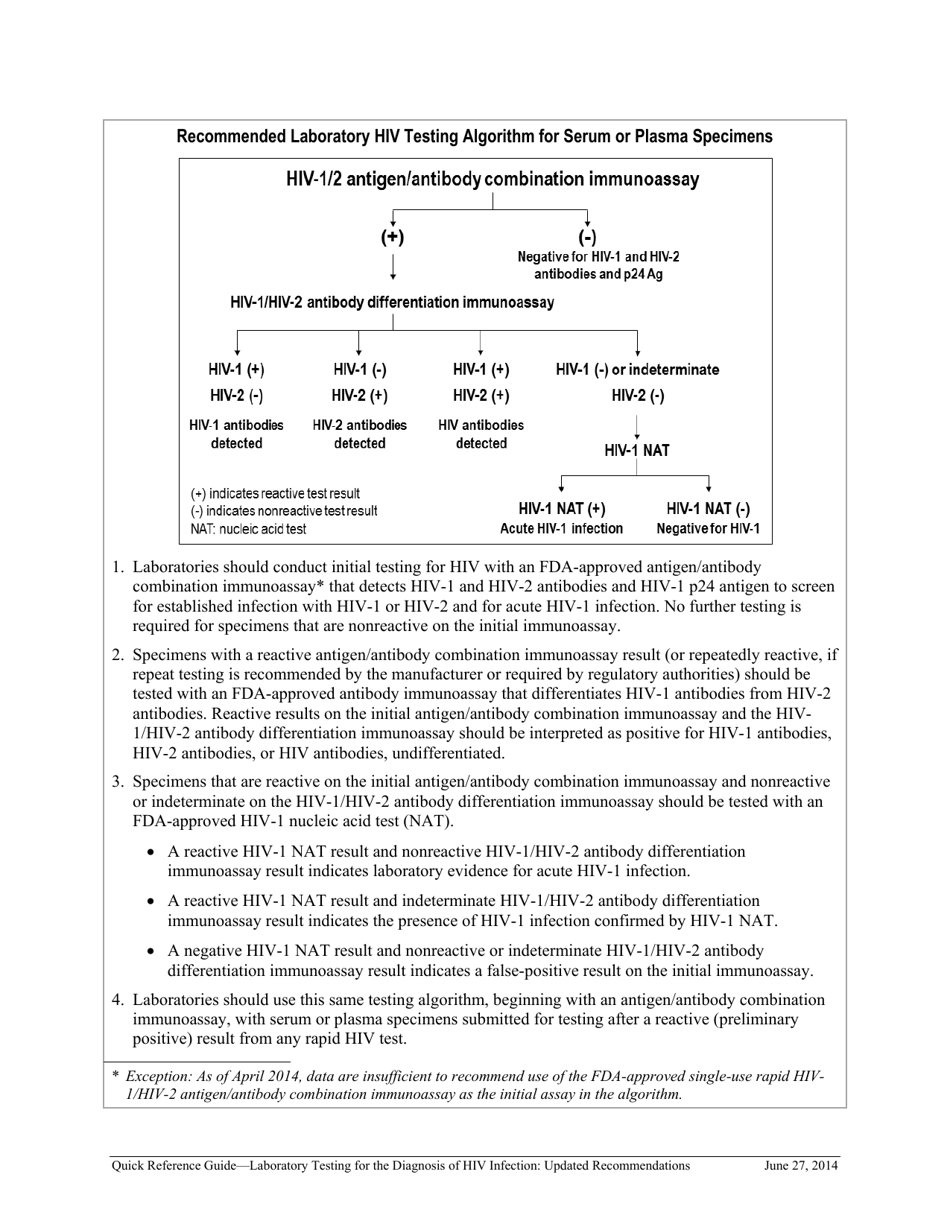
Q: What are the updated recommendations for laboratory testing for HIV?
A: The recommendations for HIV testing include using a combination of antigen/antibody tests followed by a confirmatory test.
Q: What type of tests are used for HIV testing?
A: Antigen/antibody tests and confirmatory tests are used for HIV testing.
Q: Why are antigen/antibody tests used for HIV testing?
A: Antigen/antibody tests are used because they can detect both antigens and antibodies produced by the body in response to HIV infection.
Q: What is a confirmatory test for HIV?
A: A confirmatory test is a test that is used to confirm the positive result from an initial screening test.
Q: Why is a confirmatory test necessary for HIV diagnosis?
A: A confirmatory test is necessary to rule out false-positive results and ensure accurate HIV diagnosis.
Q: How soon after exposure can HIV be detected through laboratory testing?
A: HIV can be detected through laboratory testing as early as 2-4 weeks after exposure.
Q: What are the advantages of early HIV detection through laboratory testing?
A: Early detection of HIV allows for early intervention, treatment, and prevention of transmission to others.
Q: Does a negative HIV test result always mean a person is not infected with HIV?
A: No, a negative HIV test result does not guarantee that a person is not infected with HIV, especially if the test is performed during the window period.
Q: What is the window period for HIV testing?
A: The window period is the time between HIV infection and the point when HIV can be detected by a laboratory test.
Form Details:
- The latest edition currently provided by the U.S. Department of Health and Human Services - Centers for Disease Control and Prevention;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of the form by clicking the link below or browse more legal forms and templates provided by the issuing department.