This version of the form is not currently in use and is provided for reference only. Download this version of
Form OP-UA34 (TCEQ-10291)
for the current year.
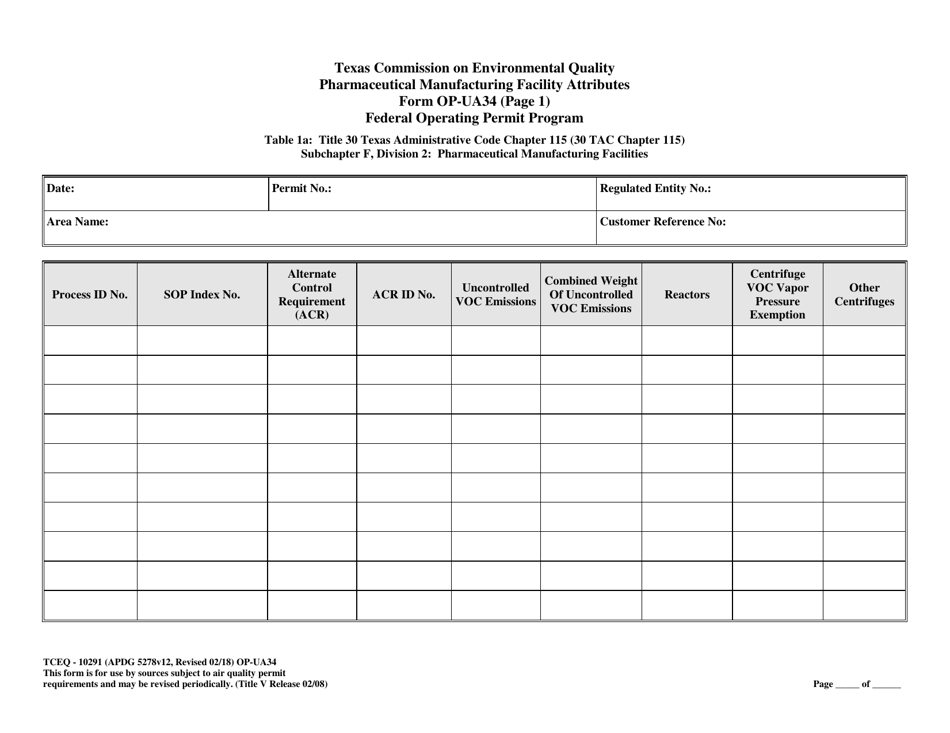
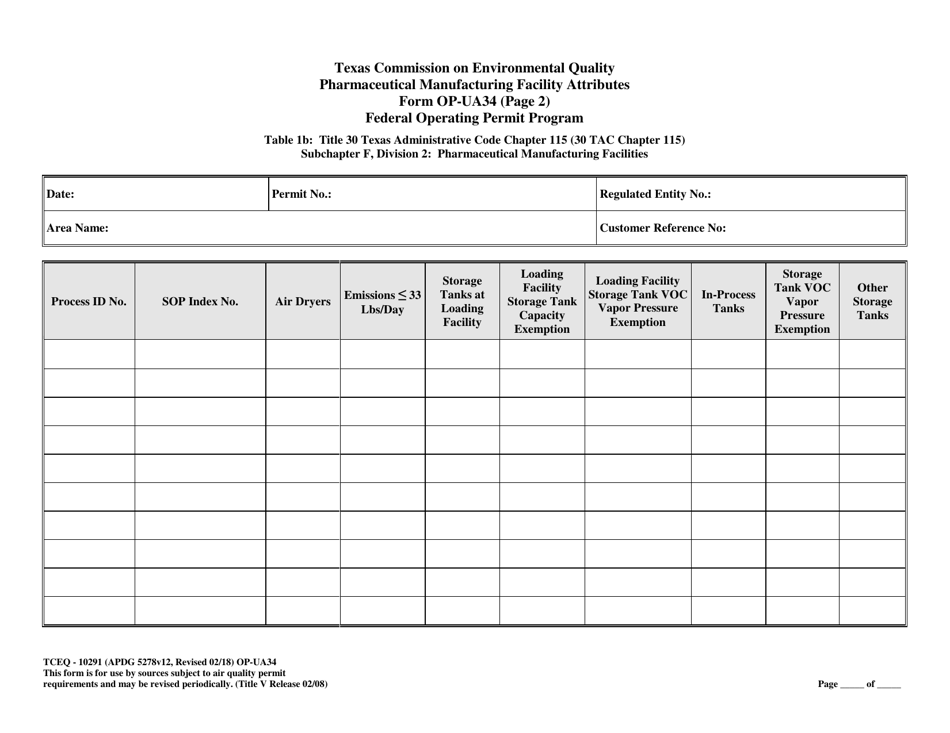
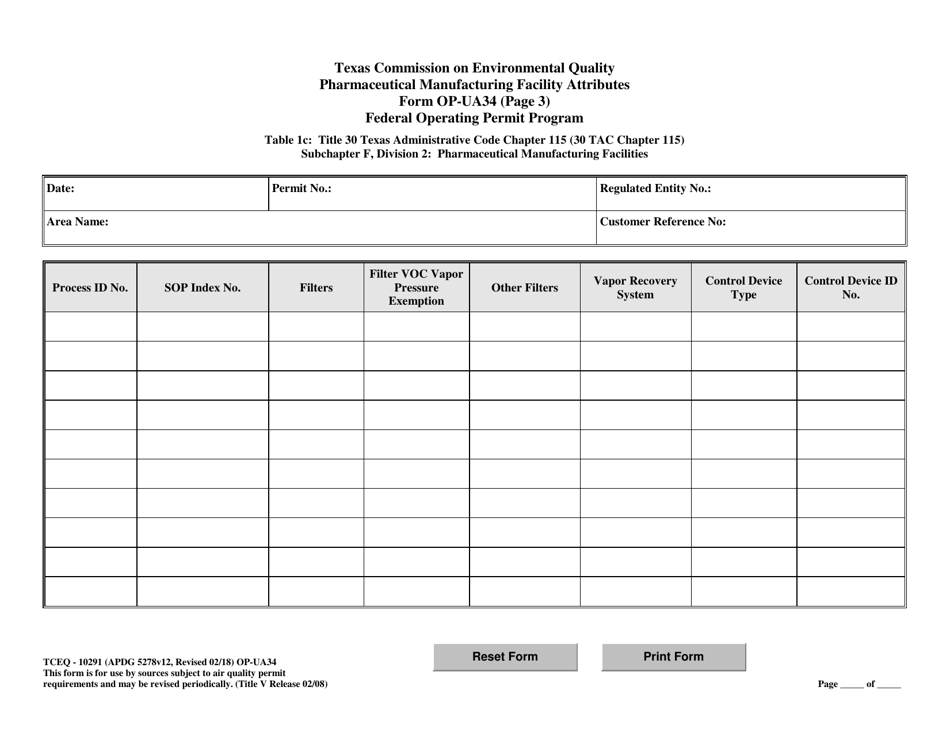
Form OP-UA34 (TCEQ-10291) Pharmaceutical Manufacturing Facility Attributes - Texas
What Is Form OP-UA34 (TCEQ-10291)?
This is a legal form that was released by the Texas Commission on Environmental Quality - a government authority operating within Texas. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form OP-UA34?
A: Form OP-UA34 is a document required by the Texas Commission on Environmental Quality (TCEQ) for pharmaceutical manufacturing facilities.
Q: What is TCEQ?
A: TCEQ stands for Texas Commission on Environmental Quality. It is the environmental agency of the state of Texas.
Q: What are pharmaceutical manufacturing facilities?
A: Pharmaceutical manufacturing facilities are facilities involved in the production of pharmaceutical drugs.
Q: What are the attributes covered in Form OP-UA34?
A: Form OP-UA34 covers the various attributes of a pharmaceutical manufacturing facility in Texas.
Q: Why is Form OP-UA34 required?
A: Form OP-UA34 is required to ensure compliance with environmental regulations and to monitor the impact of pharmaceutical manufacturing facilities on the environment.
Q: Who needs to submit Form OP-UA34?
A: Pharmaceutical manufacturing facilities in Texas need to submit Form OP-UA34 to the TCEQ.
Q: What information is required in Form OP-UA34?
A: Form OP-UA34 requires information such as facility details, manufacturing processes, emissions, waste management, and environmental controls.
Q: Are there any fees associated with Form OP-UA34?
A: Yes, there may be application fees associated with submitting Form OP-UA34. The specific fees depend on the size and type of the facility.
Q: What are the consequences of not submitting Form OP-UA34?
A: Failure to submit Form OP-UA34 or non-compliance with environmental regulations can result in penalties, fines, or legal action.
Q: Are there any exceptions to submitting Form OP-UA34?
A: There may be exceptions or exemptions for certain types or sizes of pharmaceutical manufacturing facilities. It is best to consult with the TCEQ to determine if an exemption applies.
Q: Is Form OP-UA34 specific to Texas?
A: Yes, Form OP-UA34 is specific to pharmaceutical manufacturing facilities in Texas and is required by the TCEQ.
Q: Can I get assistance in filling out Form OP-UA34?
A: Yes, the TCEQ provides assistance and guidance for filling out Form OP-UA34. You can contact them for any questions or concerns.
Q: How often do I need to submit Form OP-UA34?
A: The frequency of submitting Form OP-UA34 may vary depending on the TCEQ's requirements. It is important to check with the TCEQ for specific submission deadlines.
Q: Can I make changes to Form OP-UA34 after submission?
A: Once Form OP-UA34 is submitted, any changes or updates may require filing an amendment with the TCEQ. It is important to notify the TCEQ of any significant changes to facility attributes.
Q: Is Form OP-UA34 confidential?
A: The information submitted in Form OP-UA34 may be subject to public disclosure under the Texas Public Information Act. It is important to review the applicable laws and regulations regarding confidentiality.
Q: What is the purpose of monitoring facility attributes?
A: Monitoring facility attributes helps ensure compliance with environmental regulations, assess the impact on the environment, and promote responsible and sustainable pharmaceutical manufacturing practices.
Q: Can I consult with an environmental expert for assistance?
A: Yes, consulting with an environmental expert, such as an environmental engineer or consultant, can provide valuable guidance and expertise in filling out Form OP-UA34 and ensuring compliance with regulations.
Form Details:
- Released on February 1, 2018;
- The latest edition provided by the Texas Commission on Environmental Quality;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form OP-UA34 (TCEQ-10291) by clicking the link below or browse more documents and templates provided by the Texas Commission on Environmental Quality.