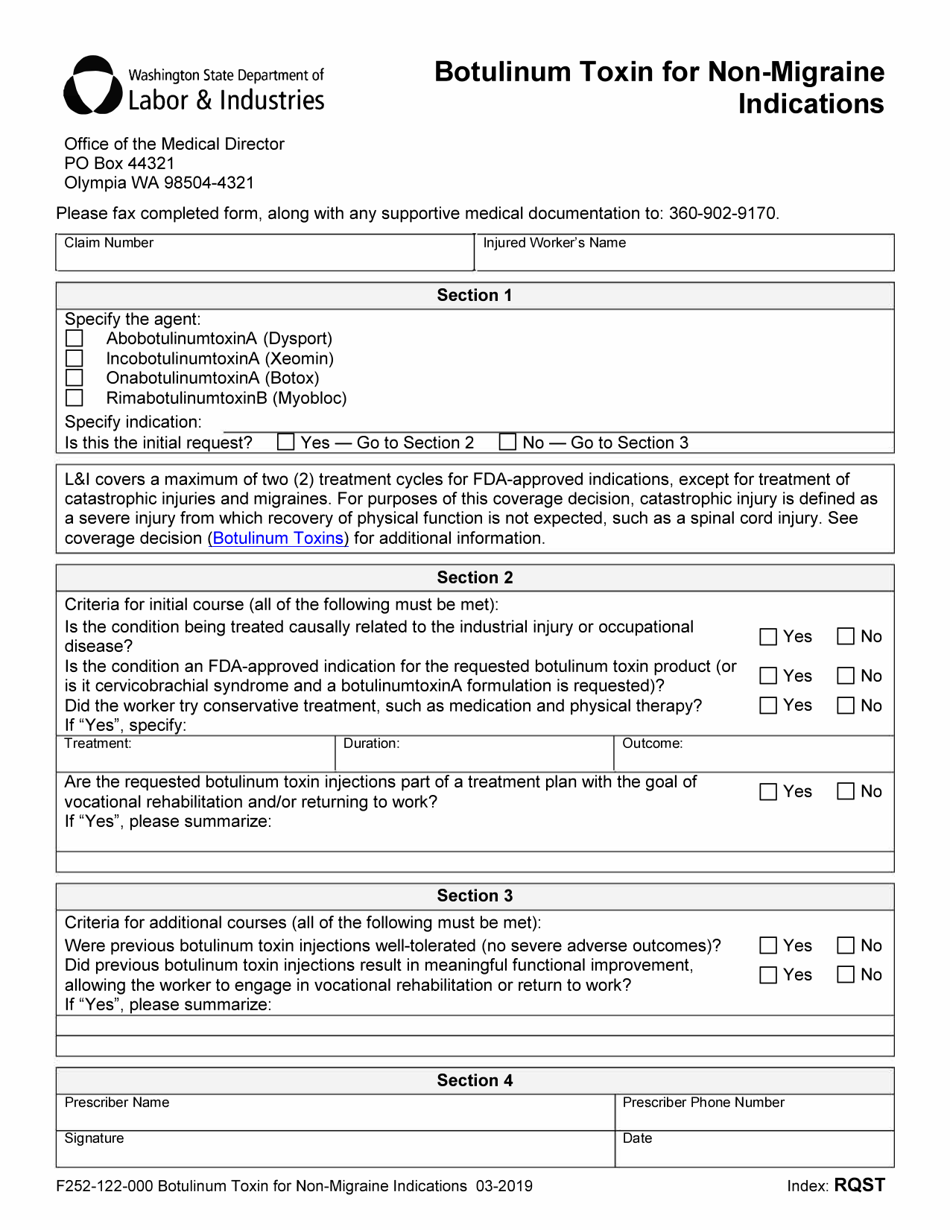
This version of the form is not currently in use and is provided for reference only. Download this version of
Form F252-122-000
for the current year.
Form F252-122-000 Botulinum Toxin for Non-migraine Indications - Washington
What Is Form F252-122-000?
This is a legal form that was released by the Washington State Department of Labor and Industries - a government authority operating within Washington. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form F252-122-000?
A: Form F252-122-000 is a document related to the administration of Botulinum Toxin for non-migraine indications in Washington.
Q: What is Botulinum Toxin?
A: Botulinum Toxin is a medication that is commonly used for cosmetic purposes, such as reducing facial wrinkles, as well as for various medical conditions.
Q: What are non-migraine indications?
A: Non-migraine indications refer to medical conditions or uses of Botulinum Toxin other than treating migraines. Examples include muscle spasms, excessive sweating, and chronic pain.
Q: What is the purpose of Form F252-122-000?
A: The purpose of Form F252-122-000 is to provide documentation and guidelines for the administration, monitoring, and reporting of Botulinum Toxin for non-migraine indications in Washington.
Q: Who needs to use Form F252-122-000?
A: Healthcare professionals who are authorized to administer Botulinum Toxin for non-migraine indications in Washington need to use Form F252-122-000.
Q: Are there any specific requirements for using Form F252-122-000?
A: Yes, there are specific requirements and guidelines outlined in the form that need to be followed for the administration, monitoring, and reporting of Botulinum Toxin for non-migraine indications.
Q: What should I do if I have questions or need assistance regarding Form F252-122-000?
A: If you have questions or need assistance regarding Form F252-122-000, you can contact the Washington State Department of Labor & Industries or consult with healthcare professionals who are familiar with the form and its requirements.
Q: Is Form F252-122-000 only applicable in Washington?
A: Yes, Form F252-122-000 is specific to Washington and is not applicable for other states or regions.
Q: Can I administer Botulinum Toxin for non-migraine indications without using Form F252-122-000?
A: No, healthcare professionals in Washington who are authorized to administer Botulinum Toxin for non-migraine indications are required to use Form F252-122-000 as part of the documentation and reporting process.
Form Details:
- Released on March 1, 2019;
- The latest edition provided by the Washington State Department of Labor and Industries;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form F252-122-000 by clicking the link below or browse more documents and templates provided by the Washington State Department of Labor and Industries.