Form F252-112-000 Direct-Acting Antiviral for Hepatitis C Prior Authorization Form - Washington
What Is Form F252-112-000?
This is a legal form that was released by the Washington State Department of Labor and Industries - a government authority operating within Washington. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is the Form F252-112-000?
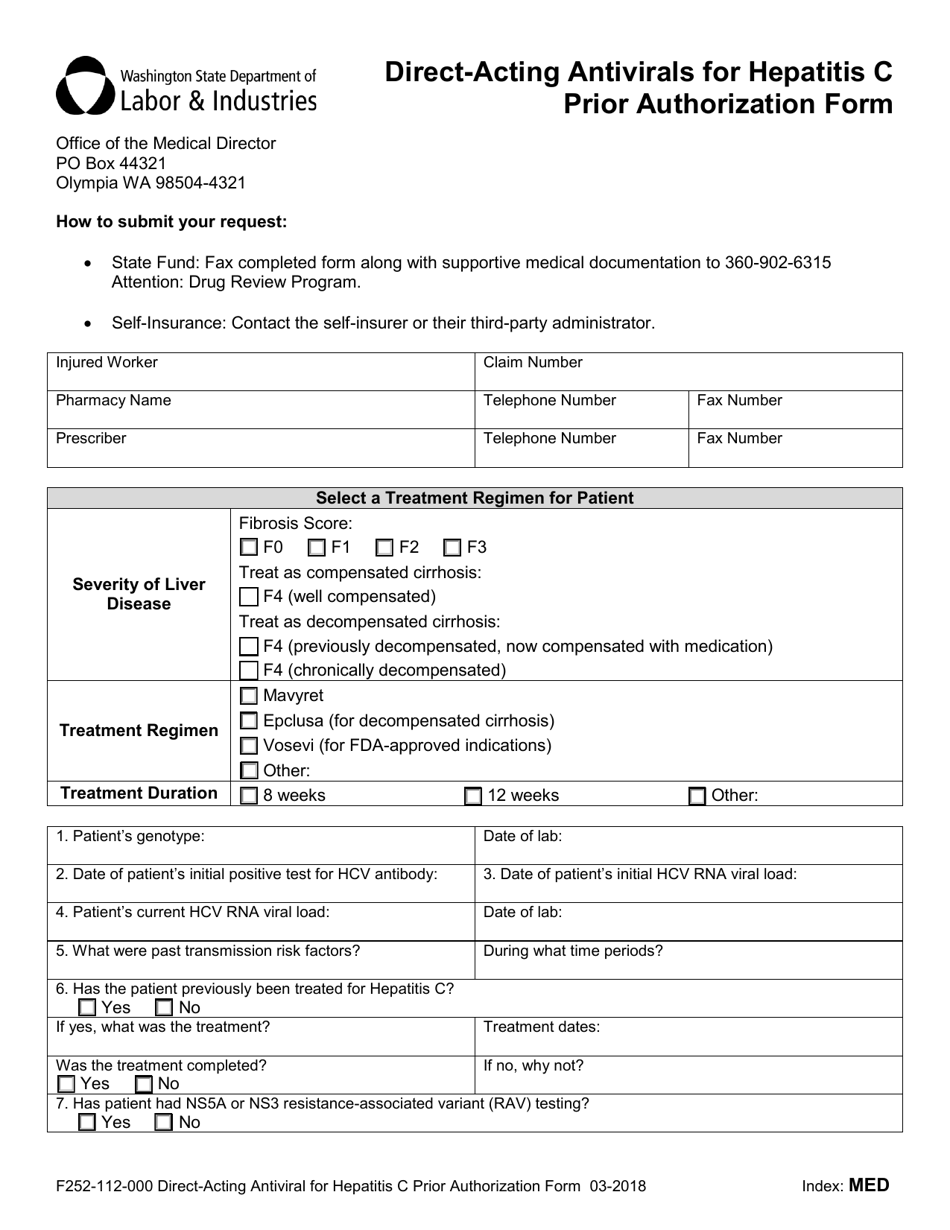
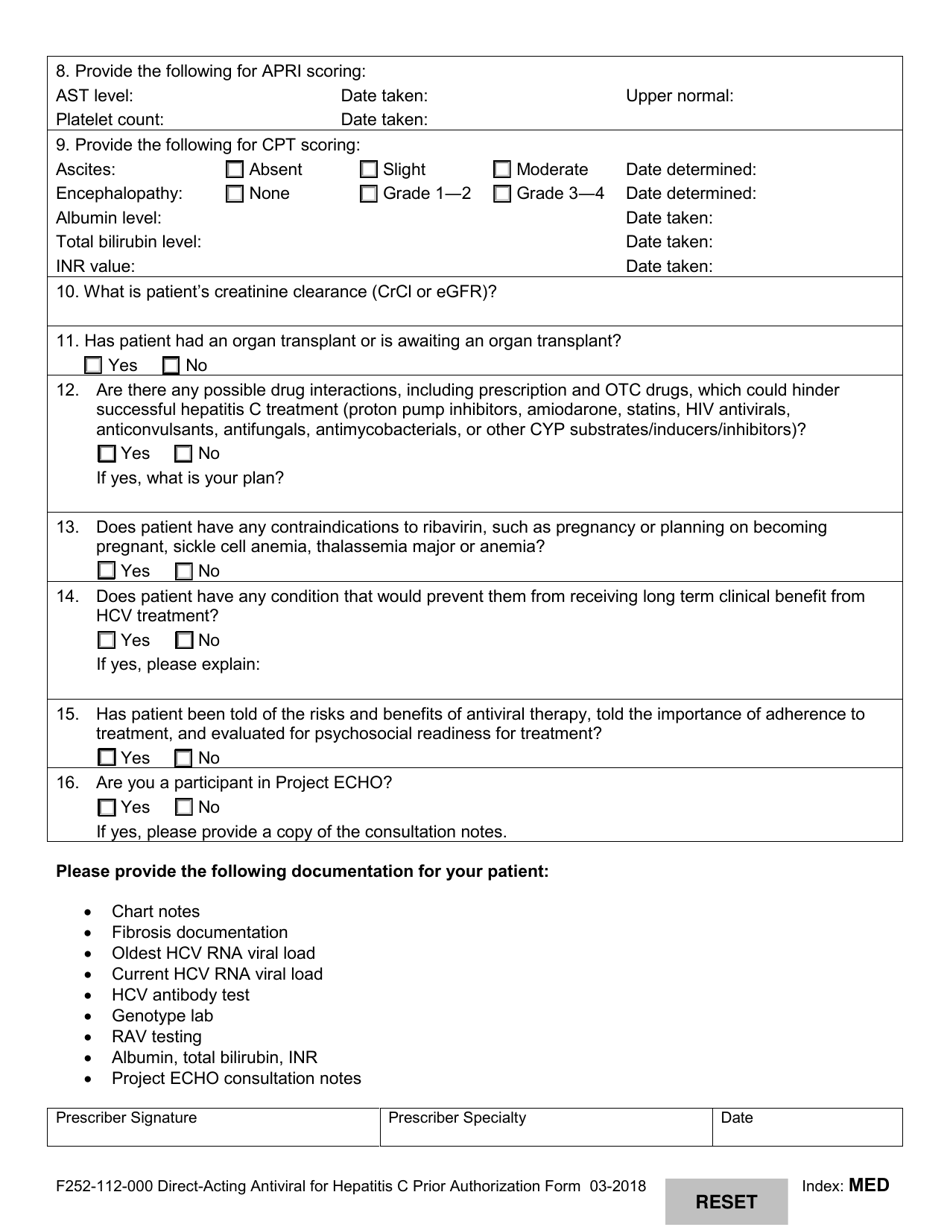
A: Form F252-112-000 is the Direct-Acting Antiviral for Hepatitis C Prior Authorization Form in Washington.
Q: What is the purpose of Form F252-112-000?
A: The purpose of Form F252-112-000 is to request prior authorization for direct-acting antiviral treatment for Hepatitis C.
Q: Who needs to fill out Form F252-112-000?
A: Healthcare providers or their authorized representatives need to fill out Form F252-112-000.
Q: What information is required on the form?
A: The form requires information about the patient, the prescriber, the treatment being requested, and the patient's medical history.
Q: Are there any fees associated with Form F252-112-000?
A: There are no fees associated with submitting Form F252-112-000.
Q: How long does it take to get a decision on the prior authorization request?
A: The decision on the prior authorization request is typically made within 48 hours of receipt of a completed form.
Form Details:
- Released on March 1, 2018;
- The latest edition provided by the Washington State Department of Labor and Industries;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form F252-112-000 by clicking the link below or browse more documents and templates provided by the Washington State Department of Labor and Industries.