This version of the form is not currently in use and is provided for reference only. Download this version of
Form FDA2541
for the current year.
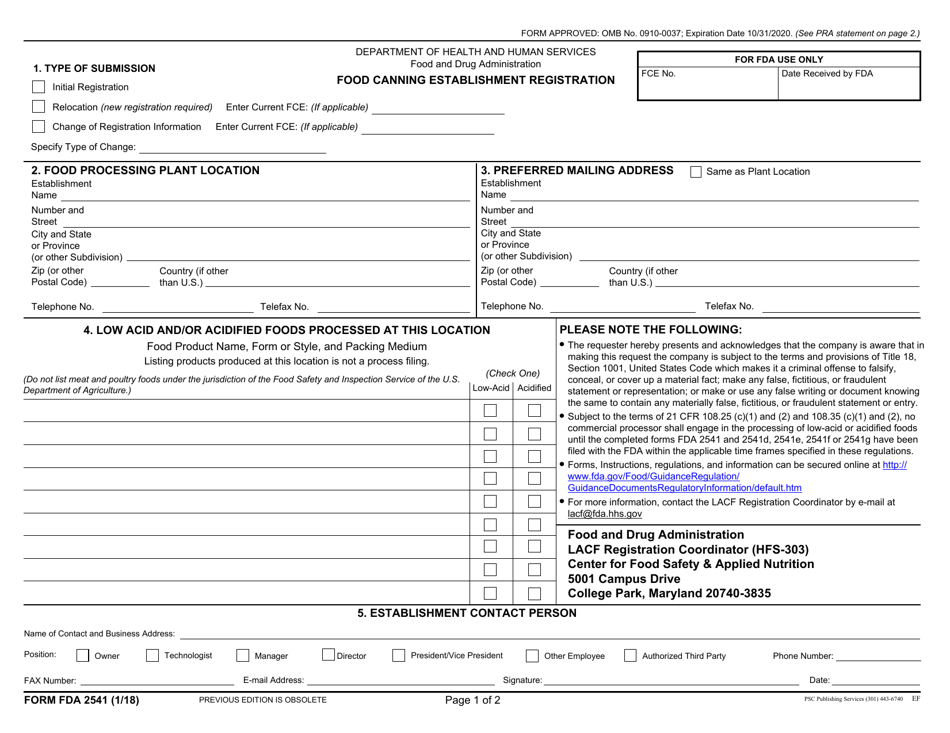
Form FDA2541 Food Canning Establishment Registration
What Is Form FDA2541?
This is a legal form that was released by the U.S. Department of Health and Human Services - U.S. Food and Drug Administration on January 1, 2018 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is FDA Form 2541?
A: FDA Form 2541 is the Food Canning Establishment Registration form.
Q: Who needs to fill out FDA Form 2541?
A: Food canning establishments must fill out FDA Form 2541.
Q: What is the purpose of FDA Form 2541?
A: The purpose of FDA Form 2541 is to register food canning establishments with the FDA.
Q: What information is required on FDA Form 2541?
A: FDA Form 2541 requires information such as the name and address of the facility, the types of products being canned, and information about the equipment used.
Q: Is there a fee to submit FDA Form 2541?
A: No, there is no fee to submit FDA Form 2541.
Q: How often does FDA Form 2541 need to be submitted?
A: FDA Form 2541 must be submitted annually, within 10 days of starting operations.
Q: What are the consequences of not submitting FDA Form 2541?
A: Failure to submit FDA Form 2541 may result in regulatory action or enforcement actions by the FDA.
Q: Is FDA Form 2541 required for all types of food canning?
A: No, FDA Form 2541 is only required for low-acid canned foods and acidified foods.
Form Details:
- Released on January 1, 2018;
- The latest available edition released by the U.S. Department of Health and Human Services - U.S. Food and Drug Administration;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form FDA2541 by clicking the link below or browse more documents and templates provided by the U.S. Department of Health and Human Services - U.S. Food and Drug Administration.