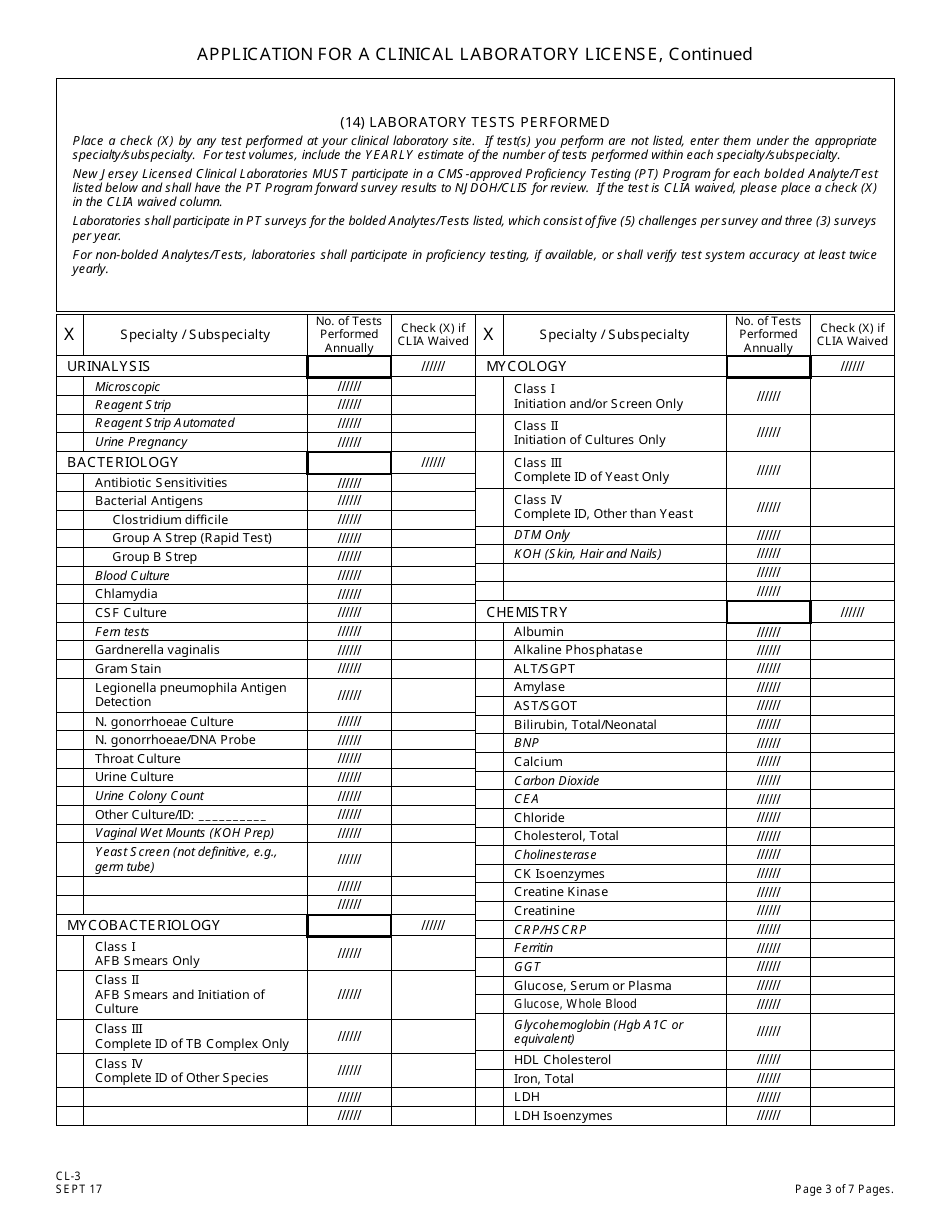
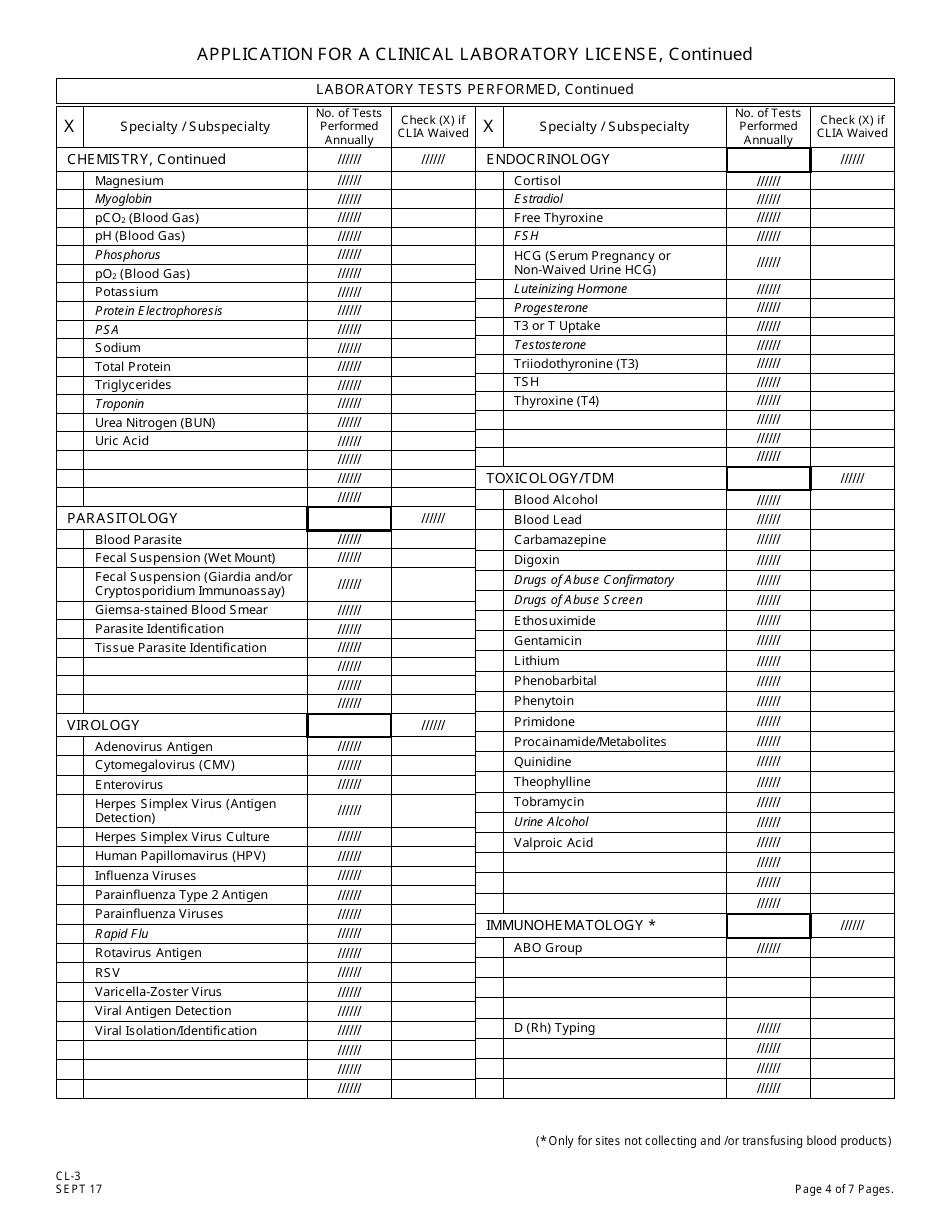
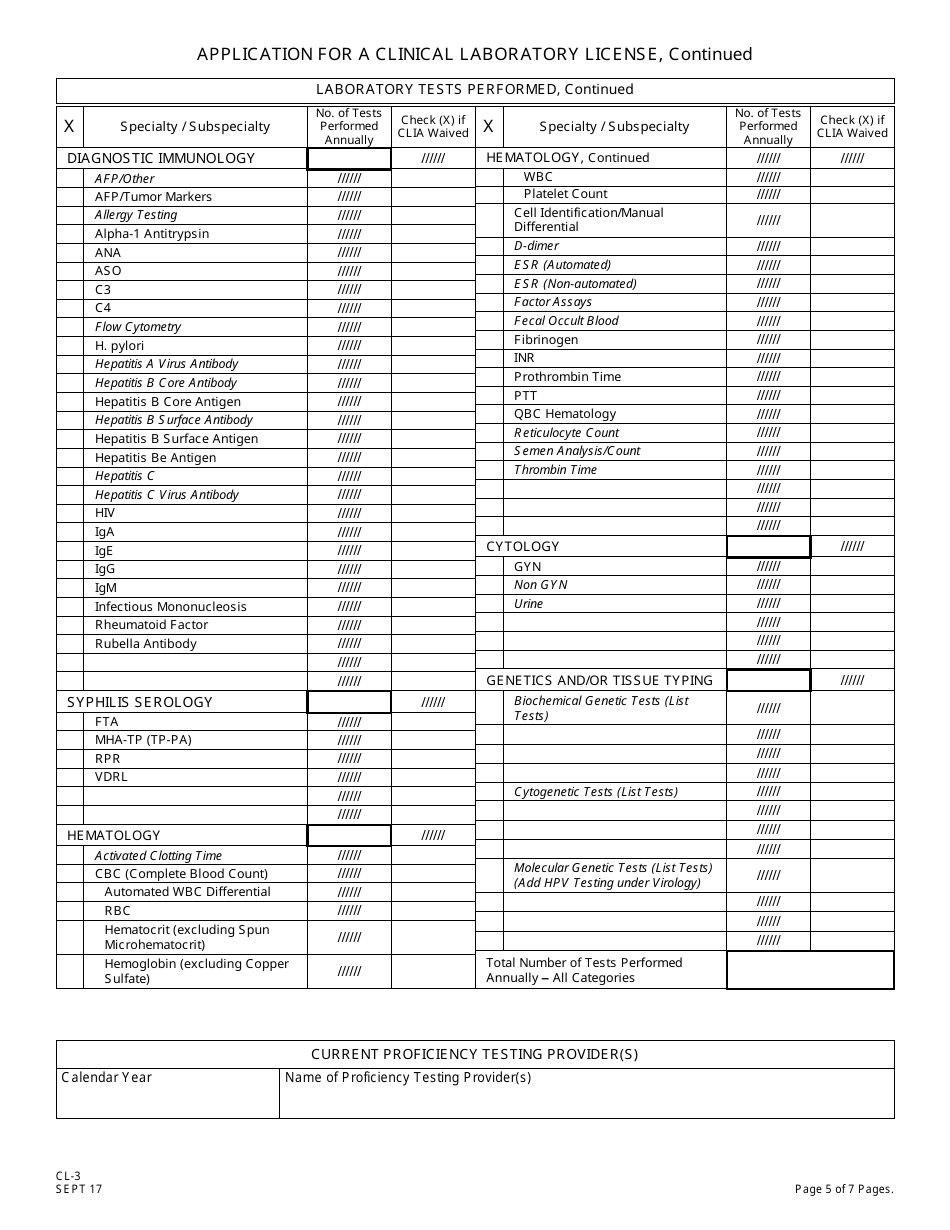
Form CL-3 Application for a Clinical Laboratory License (Clia Non-waived Tests / Onsite Testing Only) - New Jersey
What Is Form CL-3?
This is a legal form that was released by the New Jersey Department of Health - a government authority operating within New Jersey. Check the official instructions before completing and submitting the form.
FAQ
Q: What is a CL-3 Application?
A: A CL-3 Application is an application for a clinical laboratory license in New Jersey.
Q: What is a Clinical Laboratory License?
A: A Clinical Laboratory License is a permit that allows a laboratory to perform non-waived tests and onsite testing in New Jersey.
Q: What are non-waived tests?
A: Non-waived tests are laboratory tests that require more complex procedures and are typically performed in a clinical laboratory.
Q: What is onsite testing?
A: Onsite testing refers to laboratory testing that is conducted at the same location where the sample is collected.
Q: Who needs to submit a CL-3 Application?
A: Any laboratory in New Jersey that wants to perform non-waived tests and onsite testing needs to submit a CL-3 Application.
Q: How do I apply for a CL-3 Application?
A: To apply for a CL-3 Application, you need to complete and submit Form CL-3 Application for a Clinical Laboratory License (Clia Non-waived Tests/Onsite Testing Only) to the New Jersey Department of Health.
Form Details:
- Released on September 1, 2017;
- The latest edition provided by the New Jersey Department of Health;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of Form CL-3 by clicking the link below or browse more documents and templates provided by the New Jersey Department of Health.