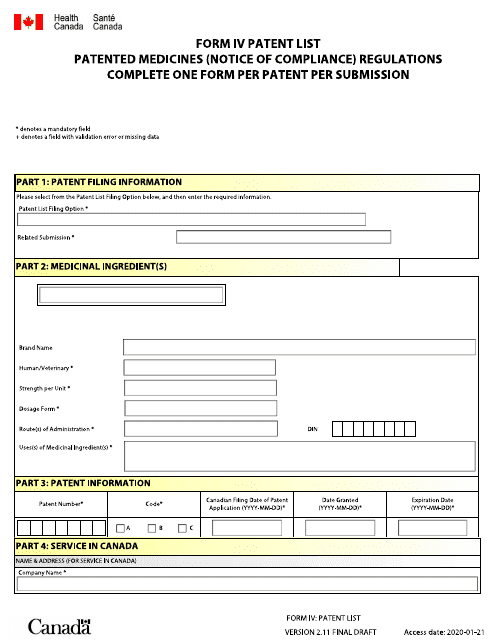
Form IV Patent List - Patented Medicines (Notice of Compliance) Regulations - Canada (English / French)
The Form IV Patent List is used in Canada to list the patents that cover specific pharmaceutical products. It is part of the Patented Medicines (Notice of Compliance) Regulations. The list can be in either English or French.
The form IV Patent List is filed by the patent holder or their authorized representative.
FAQ
Q: What is the Form IV Patent List?
A: The Form IV Patent List is a list of patented medicines in Canada.
Q: What are the Patented Medicines (Notice of Compliance) Regulations?
A: The Patented Medicines (Notice of Compliance) Regulations are regulations that govern the listing and protection of patented medicines in Canada.
Q: Why is the Form IV Patent List important?
A: The Form IV Patent List is important because it provides information on which pharmaceutical products are protected by patents in Canada.
Q: What is a Notice of Compliance?
A: A Notice of Compliance is a document issued by Health Canada indicating that a pharmaceutical product meets the regulatory requirements for sale and use in Canada.
Q: Is the Form IV Patent List available in both English and French?
A: Yes, the Form IV Patent List is available in both English and French.
Q: Who is responsible for maintaining the Form IV Patent List?
A: Health Canada is responsible for maintaining the Form IV Patent List.
Q: What happens if a generic manufacturer wants to bring a generic version of a patented medicine to the market?
A: If a generic manufacturer wants to bring a generic version of a patented medicine to the market, they are required to file an Abbreviated New Drug Submission (ANDS) and provide notice to the patentee.
Q: How long do patents listed on the Form IV Patent List last?
A: Patents listed on the Form IV Patent List last for a period of 20 years from the date of filing.
Q: Can pharmaceutical companies remove their patents from the Form IV Patent List?
A: No, pharmaceutical companies cannot remove their patents from the Form IV Patent List.
Q: How often is the Form IV Patent List updated?
A: The Form IV Patent List is updated on a regular basis as new patents are granted or expire.