Form IRB-1 Application for Initial Review - New Jersey
What Is Form IRB-1?
This is a legal form that was released by the New Jersey Department of Health - a government authority operating within New Jersey. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is an IRB-1 Application?
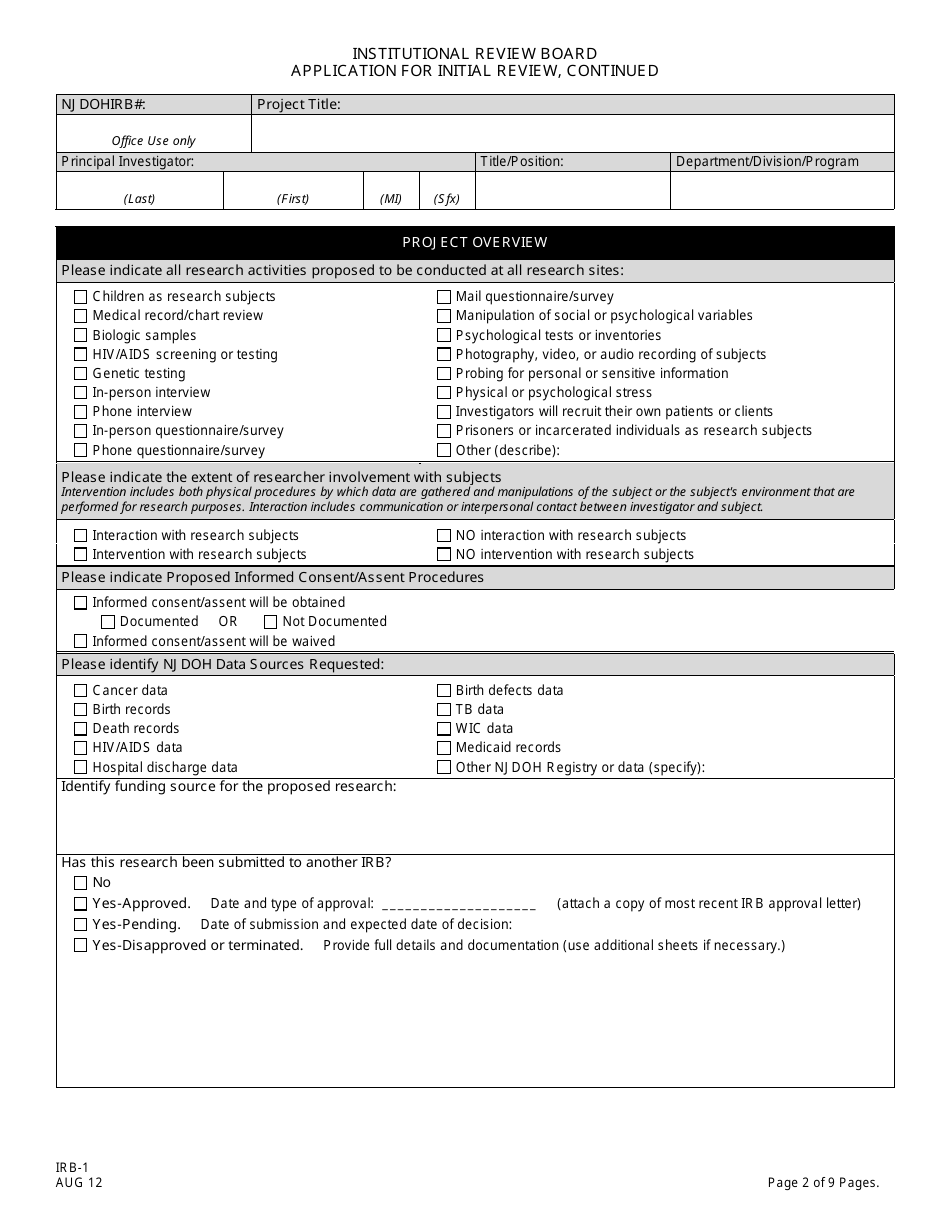
A: An IRB-1 Application is a form used for requesting an initial review of a research project by an Institutional Review Board (IRB) in New Jersey.
Q: What is the purpose of an IRB-1 Application?
A: The purpose of an IRB-1 Application is to provide information about a research project, including its ethical considerations, to an IRB for review and approval.
Q: Who needs to submit an IRB-1 Application?
A: Researchers conducting human subjects research in New Jersey, including students and faculty, need to submit an IRB-1 Application.
Q: What information is required in an IRB-1 Application?
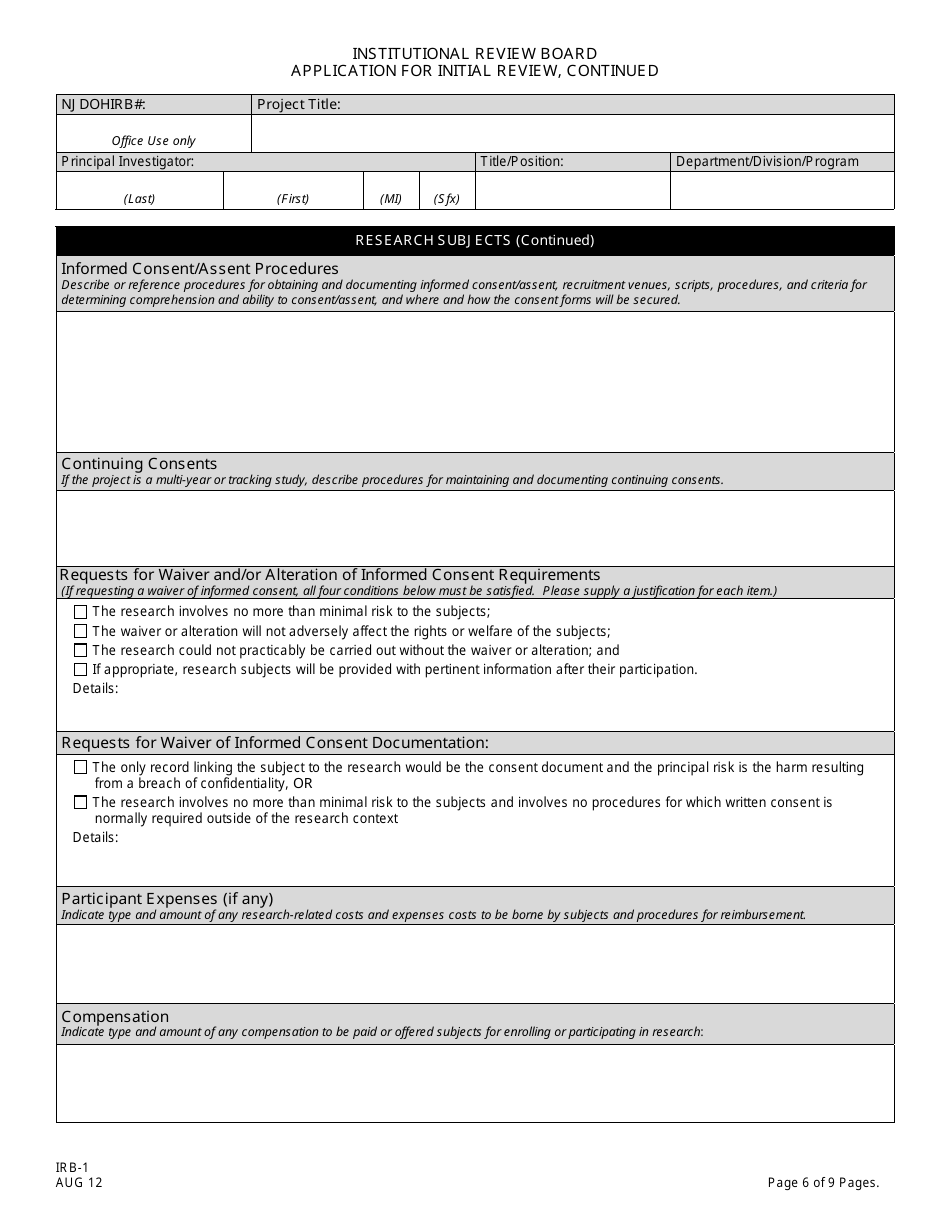
A: An IRB-1 Application requires information on the research project's purpose, procedures, risks, benefits, informed consent process, and participant selection.
Q: Are there any deadlines for submitting an IRB-1 Application?
A: Deadlines for submitting an IRB-1 Application may vary depending on the institution or organization, so it is important to check with the IRB or research office for specific deadlines.
Q: How long does it take to get IRB approval for a research project?
A: The time it takes to get IRB approval for a research project can vary, but it generally takes several weeks to a few months.
Q: What happens after submitting an IRB-1 Application?
A: After submitting an IRB-1 Application, the application will be reviewed by the IRB to ensure compliance with ethical guidelines. The researcher will receive a notification of approval or any requested modifications.
Form Details:
- Released on August 1, 2012;
- The latest edition provided by the New Jersey Department of Health;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of Form IRB-1 by clicking the link below or browse more documents and templates provided by the New Jersey Department of Health.