Documentation of Vaccine Storage Unit Temperature Monitoring - Nevada
Documentation of Vaccine Storage Unit Temperature Monitoring is a legal document that was released by the Nevada Department of Health and Human Services - a government authority operating within Nevada.
FAQ
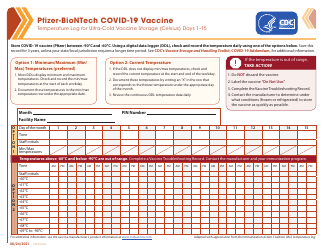
Q: What is the purpose of vaccine storage unit temperature monitoring?
A: The purpose of vaccine storage unit temperature monitoring is to ensure that vaccines are stored at the correct temperature to maintain their effectiveness and safety.
Q: Why is it important to monitor the temperature of vaccine storage units?
A: Monitoring the temperature of vaccine storage units is important to prevent the vaccines from being exposed to temperatures that are too high or too low, which can affect their potency and efficacy.
Q: What is the recommended temperature range for vaccine storage?
A: The recommended temperature range for vaccine storage is between 2°C and 8°C (36°F and 46°F).
Q: How often should vaccine storage unit temperatures be checked?
A: Vaccine storage unit temperatures should be checked twice a day, once in the morning and once in the afternoon, to ensure consistent monitoring.
Q: What should be done if the temperature of a vaccine storage unit goes outside the recommended range?
A: If the temperature of a vaccine storage unit goes outside the recommended range, the vaccines should be moved to a properly functioning unit and the issue should be addressed to prevent any potential damage to the vaccines.
Q: Who is responsible for vaccine storage unit temperature monitoring in Nevada?
A: In Nevada, vaccine storage unit temperature monitoring is the responsibility of healthcare providers, such as doctors, nurses, and pharmacists, who have been trained to handle and store vaccines properly.
Q: Are there any specific guidelines or regulations for vaccine storage unit temperature monitoring in Nevada?
A: Yes, Nevada follows the guidelines set by the Centers for Disease Control and Prevention (CDC) for vaccine storage and handling, which includes temperature monitoring requirements.
Q: What should be done if there is a power outage or equipment failure affecting the vaccine storage unit?
A: If there is a power outage or equipment failure affecting the vaccine storage unit, healthcare providers should follow the contingency plans and procedures in place to ensure the proper storage of vaccines during such events.
Q: Are there any temperature monitoring devices or systems recommended for vaccine storage units?
A: Yes, there are temperature monitoring devices and systems available that can provide continuous monitoring and alerts for vaccine storage units. These devices can help healthcare providers quickly identify and address any temperature fluctuations.
Q: Can vaccines be used if the temperature of the storage unit has been compromised?
A: If the temperature of the storage unit has been compromised and the vaccines have been exposed to temperatures outside the recommended range, it is recommended not to use the vaccines and to consult with the appropriate authorities or vaccine manufacturers for guidance.
Form Details:
- The latest edition currently provided by the Nevada Department of Health and Human Services;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a printable version of the form by clicking the link below or browse more documents and templates provided by the Nevada Department of Health and Human Services.