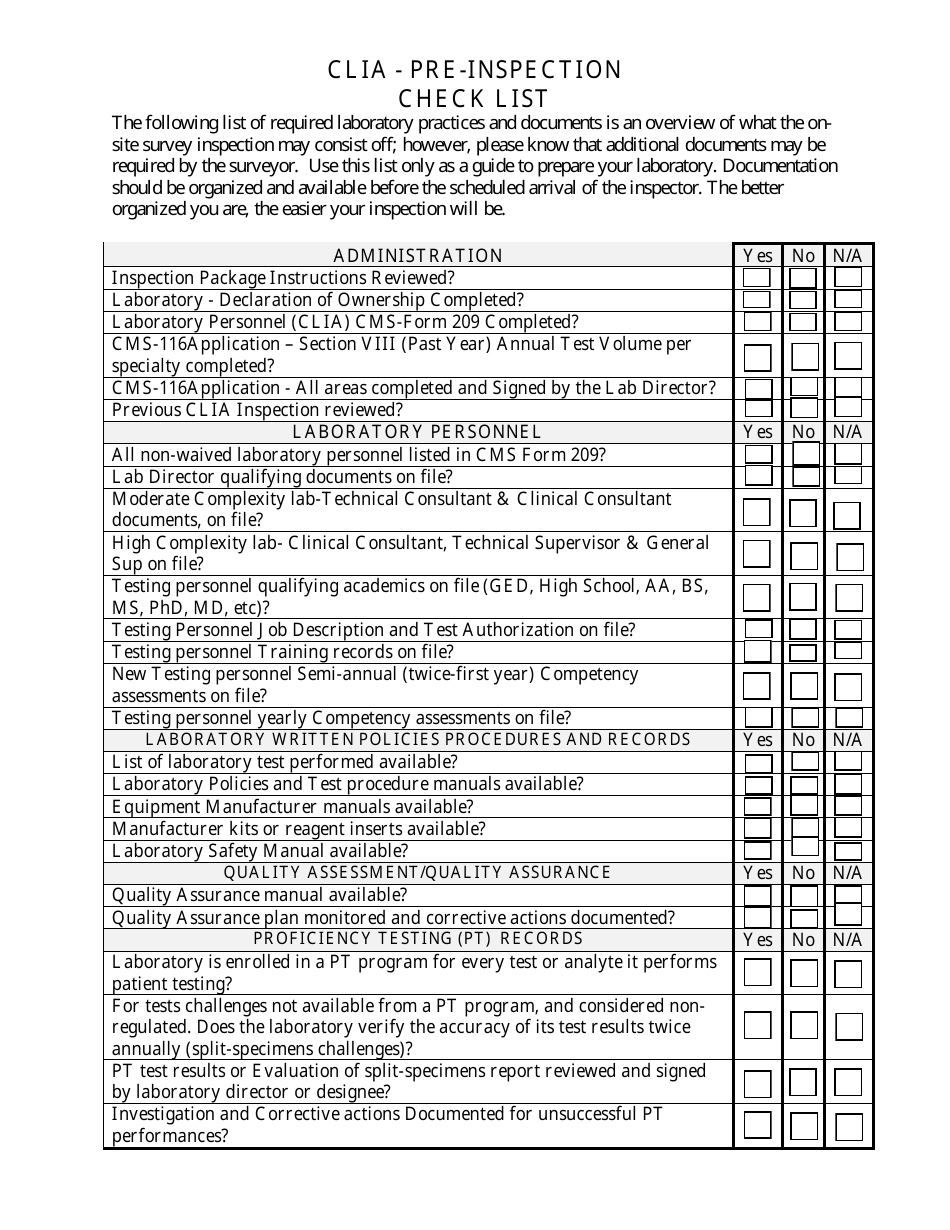
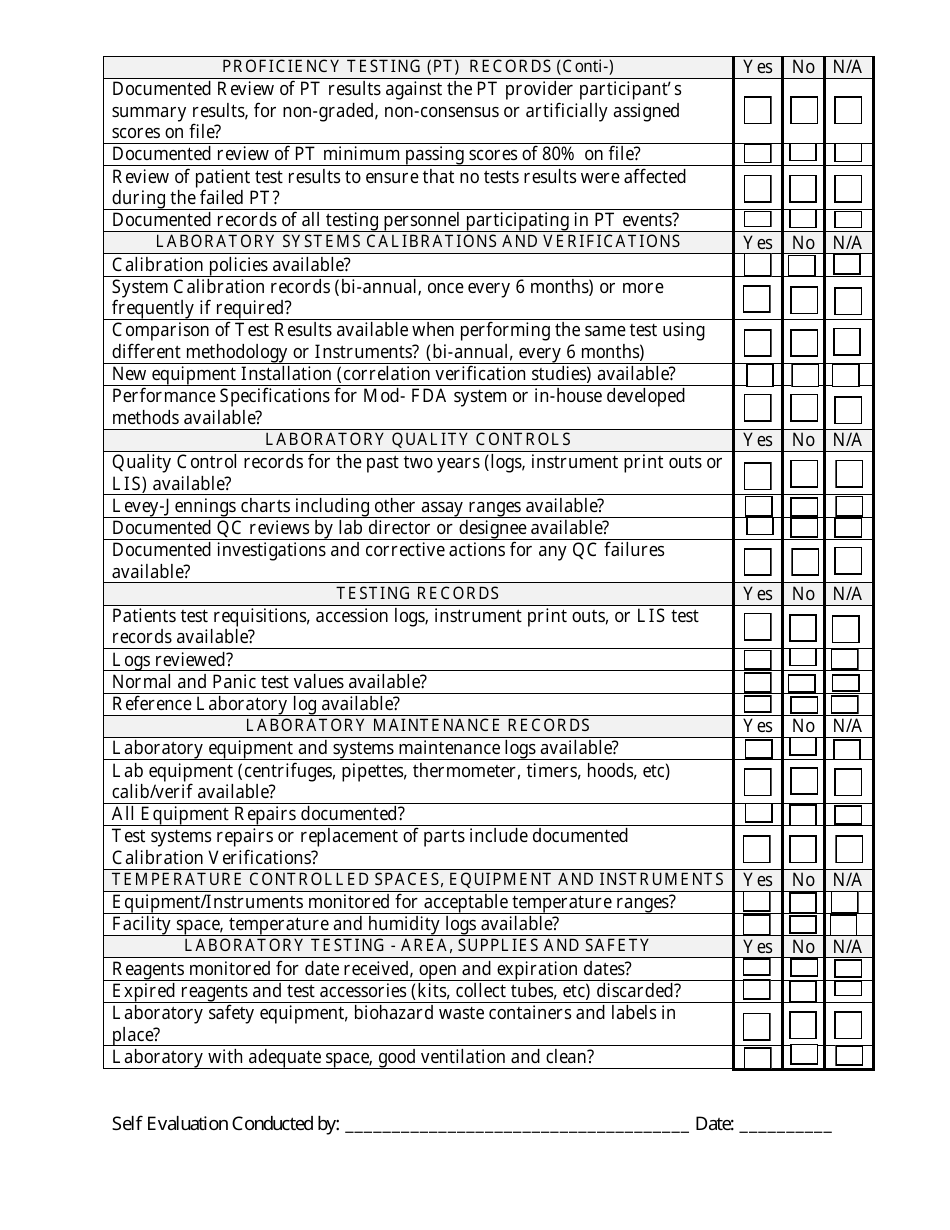
Clia - Pre-inspection Check List - Illinois
Clia - Pre-inspection Check List is a legal document that was released by the Illinois Department of Public Health - a government authority operating within Illinois.
FAQ
Q: What is the purpose of the CLIA pre-inspection checklist?
A: The CLIA pre-inspection checklist is used to ensure that laboratories in Illinois are in compliance with CLIA regulations before an inspection.
Q: What is CLIA?
A: CLIA stands for Clinical Laboratory Improvement Amendments, which are federal regulatory standards that apply to clinical laboratories to ensure quality and accuracy of laboratory testing.
Q: What is the purpose of CLIA regulations?
A: The purpose of CLIA regulations is to ensure that laboratories produce accurate and reliable test results, which are essential for patient care and public health.
Q: Who needs to comply with CLIA regulations?
A: All laboratories in the United States that perform diagnostic testing on specimens derived from humans, including those in Illinois, must comply with CLIA regulations.
Q: What does the pre-inspection checklist include?
A: The CLIA pre-inspection checklist includes a comprehensive list of items that need to be addressed before an inspection, such as personnel qualifications, quality control procedures, and laboratory safety measures.
Q: What happens if a laboratory fails to comply with CLIA regulations?
A: If a laboratory fails to comply with CLIA regulations, they may face penalties, such as monetary fines, loss of certification, or restrictions on their laboratory operations.
Form Details:
- The latest edition currently provided by the Illinois Department of Public Health;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of the form by clicking the link below or browse more documents and templates provided by the Illinois Department of Public Health.