Application for Clinical Laboratory Licensure, Registration and Approval - Connecticut
Application for Clinical Laboratory Licensure, Registration and Approval is a legal document that was released by the Connecticut State Department of Public Health - a government authority operating within Connecticut.
FAQ
Q: What is the purpose of the application?
A: The application is used to obtain clinical laboratory licensure, registration, and approval in Connecticut.
Q: Who needs to submit this application?
A: Any clinical laboratory in Connecticut that wishes to operate must submit this application.
Q: What types of laboratories require licensure, registration, and approval?
A: Clinical laboratories that perform diagnostic testing on human specimens for the purpose of assessment, prevention, or treatment of disease.
Q: What kind of information is required on the application?
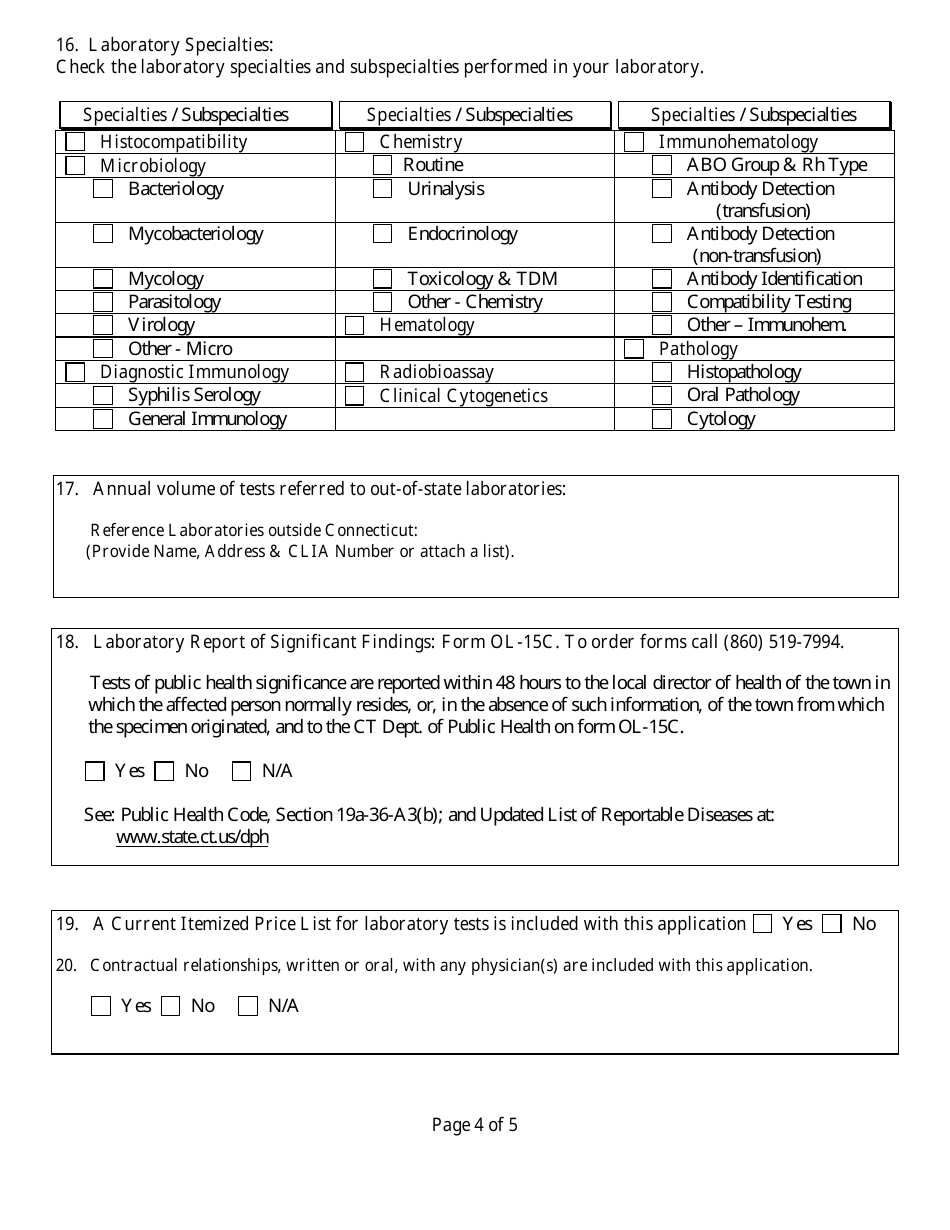
A: The application requires information about the laboratory's ownership, personnel, testing procedures, quality control, and proficiency testing.
Q: Are there any fees associated with the application?
A: Yes, there are fees associated with the application. The fees are based on the laboratory's testing volume.
Q: How long does it take to process the application?
A: The processing time for the application varies, but it typically takes several weeks to several months.
Q: What happens after the application is approved?
A: Once the application is approved, the laboratory will receive a license, registration, or approval to operate in Connecticut.
Form Details:
- Released on January 26, 2010;
- The latest edition currently provided by the Connecticut State Department of Public Health;
- Ready to use and print;
- Easy to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of the form by clicking the link below or browse more documents and templates provided by the Connecticut State Department of Public Health.