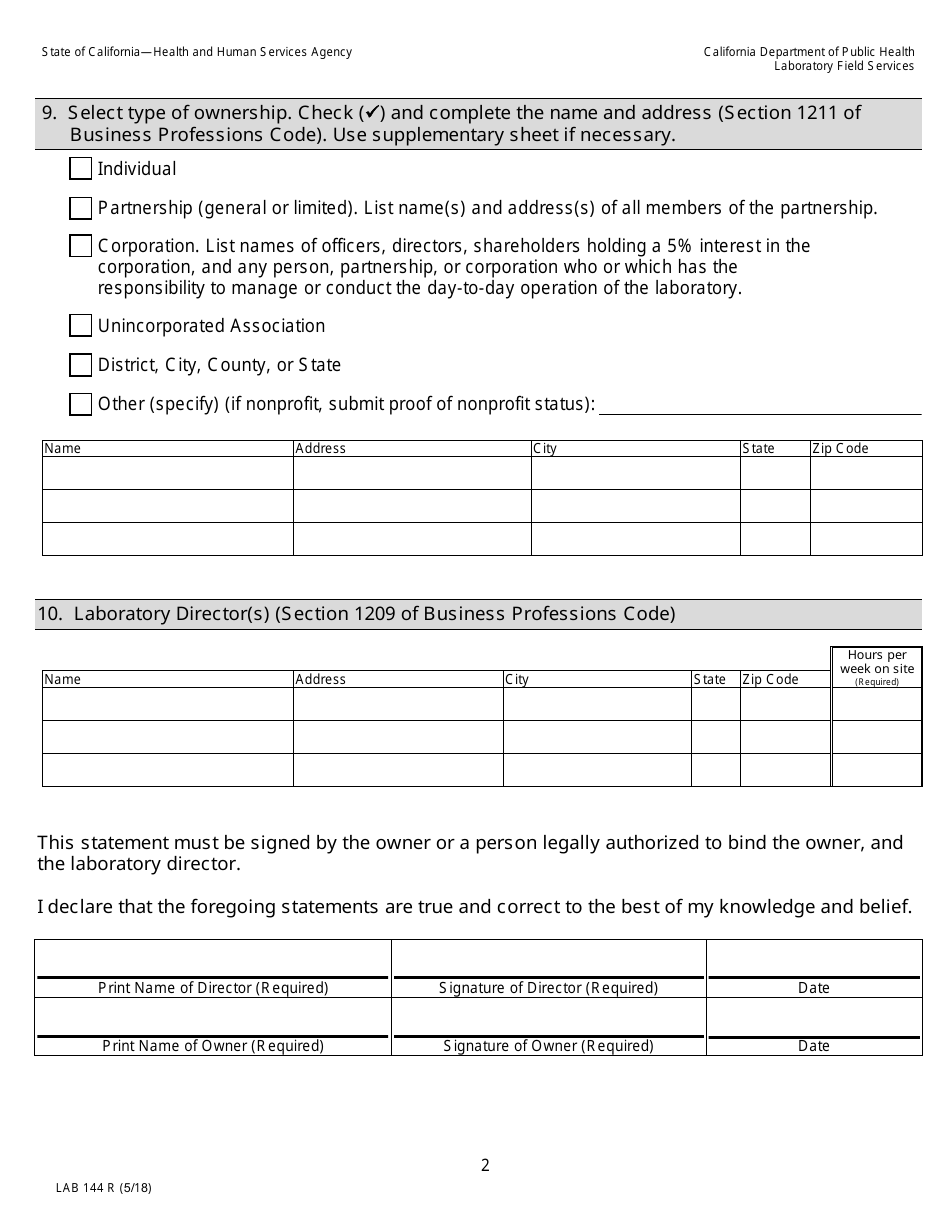
Form LAB144 R Application for Renewal Clinical Laboratory License - California
What Is Form LAB144 R?
This is a legal form that was released by the California Department of Public Health - a government authority operating within California. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is form LAB144 R?
A: Form LAB144 R is an application for the renewal of a Clinical Laboratory License in California.
Q: Who needs to complete form LAB144 R?
A: Clinical laboratories in California need to complete form LAB144 R for license renewal.
Q: What is the purpose of form LAB144 R?
A: The purpose of form LAB144 R is to apply for the renewal of a Clinical Laboratory License in California.
Q: What information is required on form LAB144 R?
A: Form LAB144 R requires information about the clinical laboratory, contact details, and payment information.
Q: Is there a fee for submitting form LAB144 R?
A: Yes, there is a fee associated with submitting form LAB144 R.
Q: When should form LAB144 R be submitted?
A: Form LAB144 R should be submitted prior to the expiration of the current Clinical Laboratory License.
Q: Are there any additional documents required with form LAB144 R?
A: Depending on the specific requirements, additional documents may be required along with form LAB144 R.
Q: How long does it take to process form LAB144 R?
A: The processing time for form LAB144 R may vary, and it is advisable to submit the application well in advance.
Q: What happens after submitting form LAB144 R?
A: After submitting form LAB144 R, the California Department of Public Health will review the application and issue a renewed Clinical Laboratory License if approved.
Form Details:
- Released on May 1, 2018;
- The latest edition provided by the California Department of Public Health;
- Easy to use and ready to print;
- Quick to customize;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form LAB144 R by clicking the link below or browse more documents and templates provided by the California Department of Public Health.