Form SF-541 Medical Record - Gynecologic Cytology
What Is Form SF-541?
This is a legal form that was released by the U.S. General Services Administration on November 1, 1977 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form SF-541?
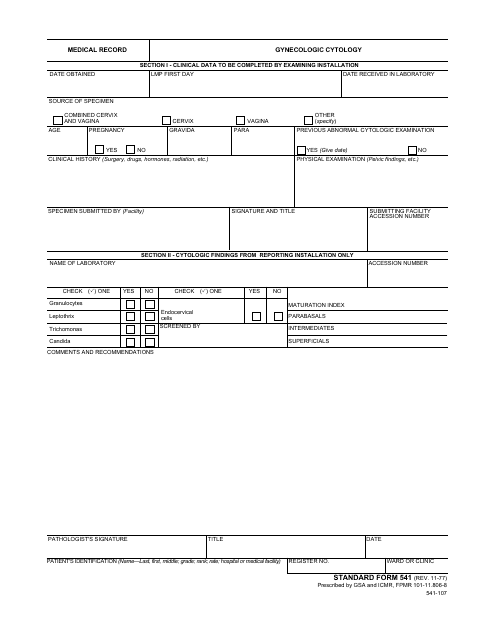
A: Form SF-541 is a medical record specifically for gynecologic cytology.
Q: Who uses Form SF-541?
A: Form SF-541 is used by healthcare professionals and facilities, such as gynecologists and laboratories, to document and track gynecologic cytology test results.
Q: What is gynecologic cytology?
A: Gynecologic cytology is the study of cells collected from the cervix or vagina to screen for abnormalities or diagnose conditions such as cervical cancer.
Q: What information is included in Form SF-541?
A: Form SF-541 includes patient information, details of the cytology test, results, and other relevant information such as previous test dates and medical history.
Q: Is Form SF-541 specific to the United States and Canada?
A: Yes, Form SF-541 is used in both the United States and Canada for documenting gynecologic cytology.
Form Details:
- Released on November 1, 1977;
- The latest available edition released by the U.S. General Services Administration;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form SF-541 by clicking the link below or browse more documents and templates provided by the U.S. General Services Administration.