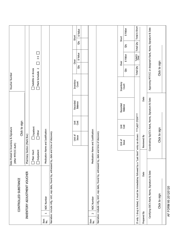
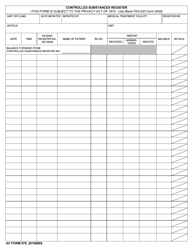
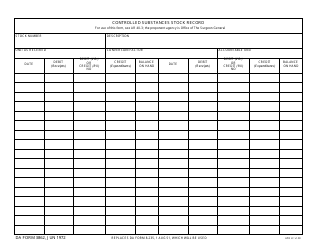
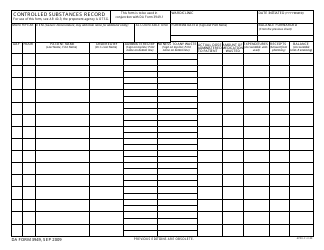
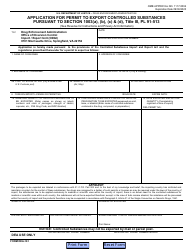
Form NIH2765-1 Request for Controlled Substances for Nonhuman Use
What Is Form NIH2765-1?
This is a legal form that was released by the U.S. Department of Health and Human Services - National Institutes of Health on December 1, 2013 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is NIH2765-1?
A: NIH2765-1 is a form used to request controlled substances for nonhuman use.
Q: Who uses NIH2765-1?
A: Researchers or institutions who need to obtain controlled substances for nonhuman research use.
Q: What is the purpose of completing NIH2765-1?
A: The form is used to ensure proper record-keeping and compliance with regulations when acquiring controlled substances for nonhuman research use.
Q: What information is required on NIH2765-1?
A: The form requires information such as the requester's name, institution, detailed substance information, and research protocol details.
Q: Are there any fees associated with filing NIH2765-1?
A: There may be fees associated with filing NIH2765-1, depending on the regulatory authority and specific circumstances. It is recommended to consult the appropriate authority for fee information.
Q: How long does it take to process NIH2765-1?
A: The processing time for NIH2765-1 can vary depending on the regulatory authority and factors such as completeness of the form and any additional requirements. It is advisable to submit the form well in advance of the anticipated need.
Form Details:
- Released on December 1, 2013;
- The latest available edition released by the U.S. Department of Health and Human Services - National Institutes of Health;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form NIH2765-1 by clicking the link below or browse more documents and templates provided by the U.S. Department of Health and Human Services - National Institutes of Health.