This version of the form is not currently in use and is provided for reference only. Download this version of
Form CMS-847
for the current year.
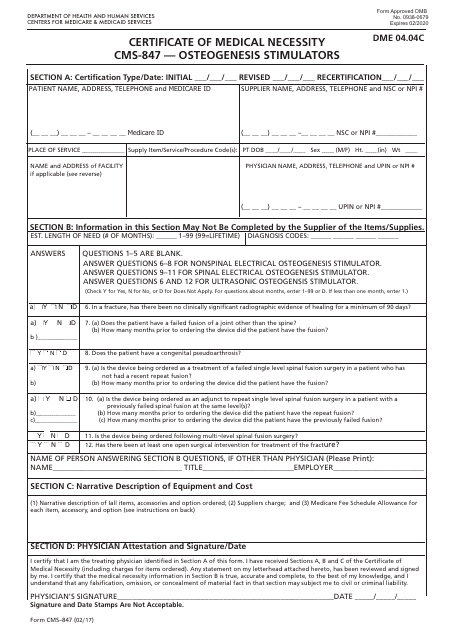
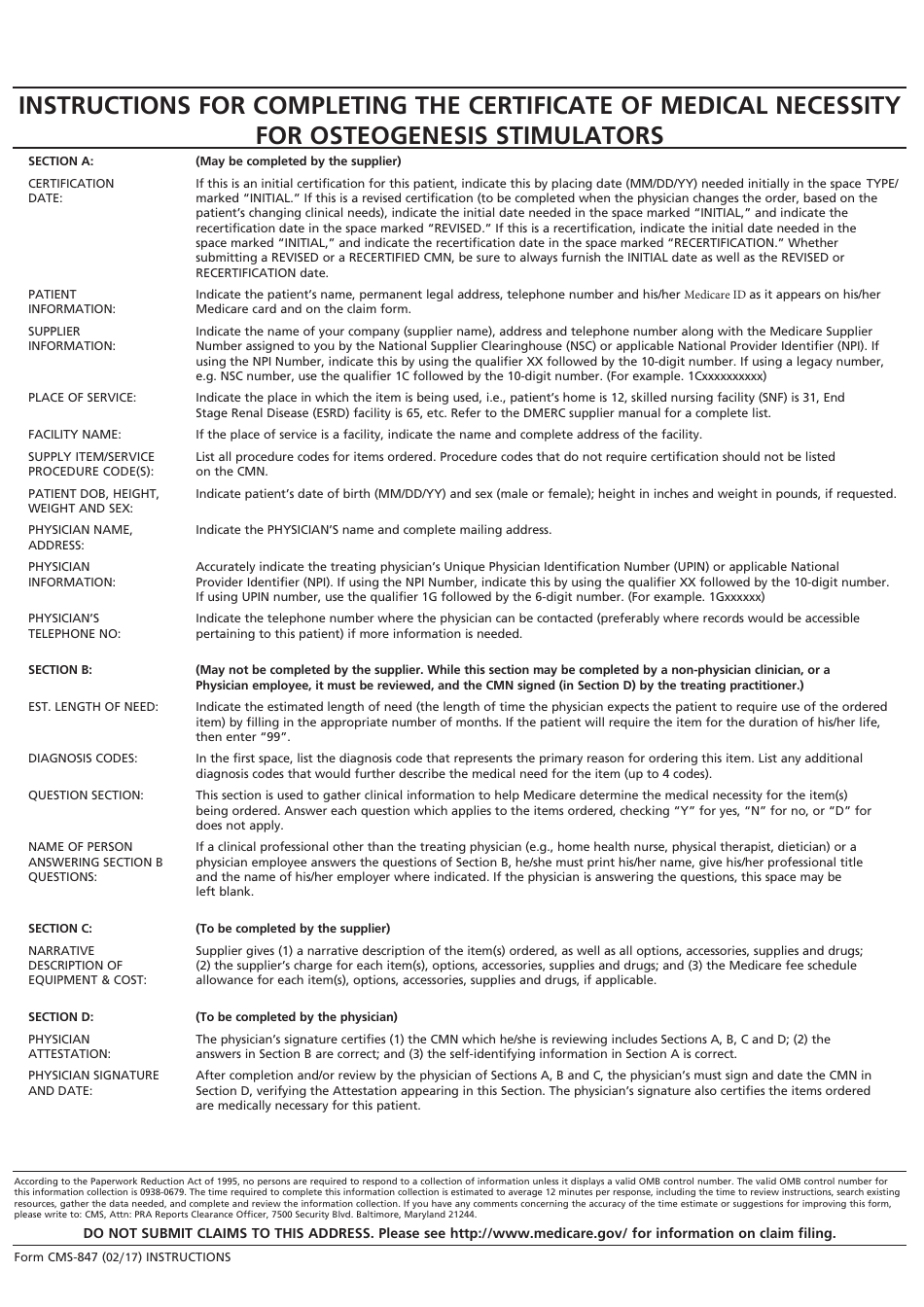
Form CMS-847 Certificate of Medical Necessity - Osteogenesis Stimulators
What Is Form CMS-847?
This is a legal form that was released by the U.S. Department of Health and Human Services - Centers for Medicare and Medicaid Services on February 1, 2017 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form CMS-847?
A: Form CMS-847 is the Certificate of Medical Necessity for Osteogenesis Stimulators.
Q: What is an Osteogenesis Stimulator?
A: An Osteogenesis Stimulator is a medical device used to promote bone healing and treat certain bone conditions.
Q: Who needs to fill out Form CMS-847?
A: The healthcare provider who is prescribing or ordering the Osteogenesis Stimulator needs to fill out the form.
Q: Why is Form CMS-847 important?
A: Form CMS-847 is important because it documents the medical necessity of the Osteogenesis Stimulator and helps determine if it will be covered by insurance.
Q: Are there any fees for submitting Form CMS-847?
A: No, there are no fees for submitting Form CMS-847.
Q: How long does it take to process Form CMS-847?
A: The processing time for Form CMS-847 varies, but it typically takes a few weeks to receive a decision from the insurance provider.
Q: What should I do if my Form CMS-847 is denied?
A: If your Form CMS-847 is denied, you can appeal the decision with your insurance provider.
Q: Can I use Form CMS-847 for other medical devices?
A: No, Form CMS-847 is specifically for Osteogenesis Stimulators and cannot be used for other medical devices.
Form Details:
- Released on February 1, 2017;
- The latest available edition released by the U.S. Department of Health and Human Services - Centers for Medicare and Medicaid Services;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form CMS-847 by clicking the link below or browse more documents and templates provided by the U.S. Department of Health and Human Services - Centers for Medicare and Medicaid Services.