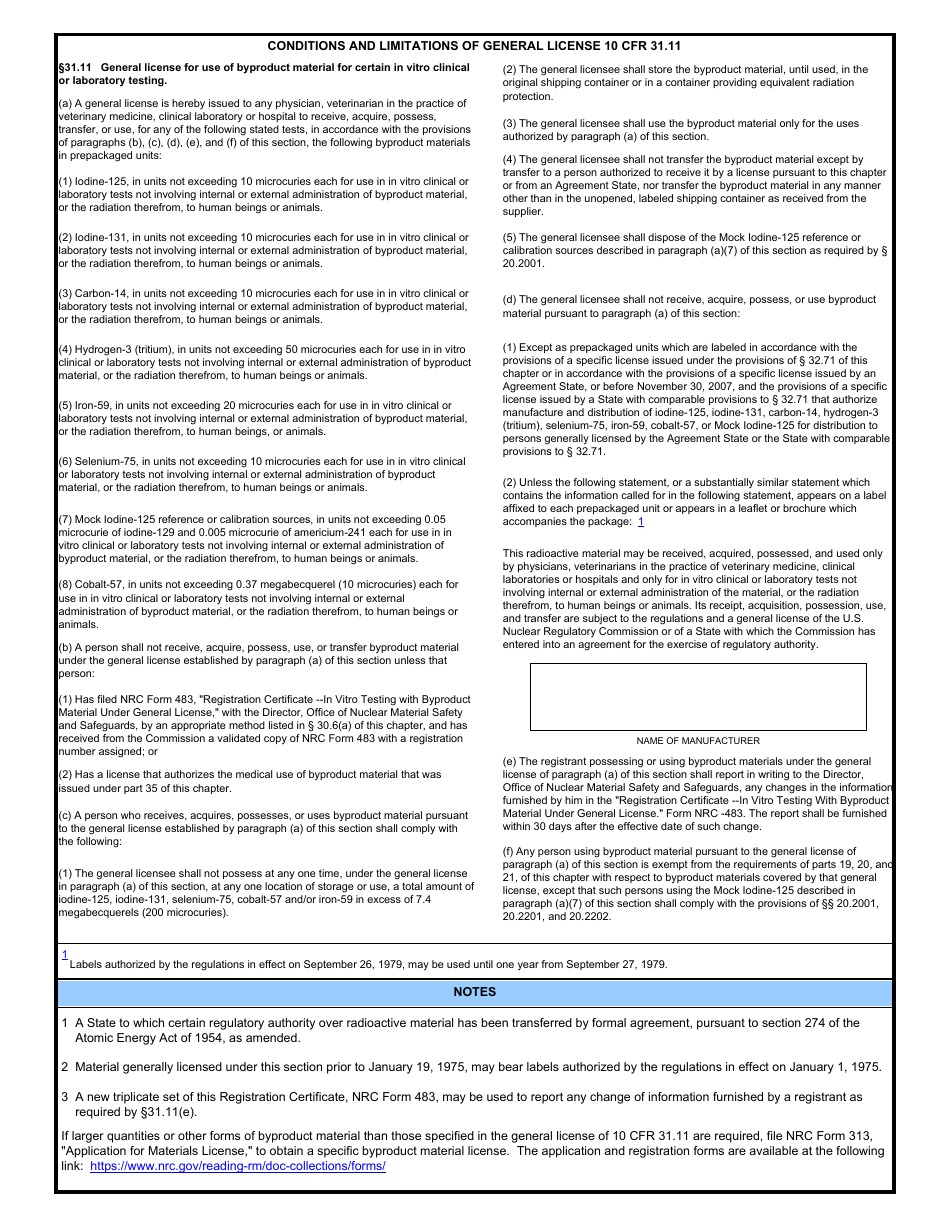
This version of the form is not currently in use and is provided for reference only. Download this version of
NRC Form 483
for the current year.
NRC Form 483 Registration Certificate - in Vitro Testing With Byproduct Material Under General License
What Is NRC Form 483?
This is a legal form that was released by the U.S. Nuclear Regulatory Commission on April 1, 2018 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is an NRC Form 483?
A: NRC Form 483 is a registration certificate.
Q: What does the NRC registration certificate allow?
A: It allows for in vitro testing with byproduct material.
Q: What is meant by in vitro testing?
A: In vitro testing refers to testing done outside the living organism, typically in a laboratory setting.
Q: What is byproduct material?
A: Byproduct material refers to any radioactive material produced during the operation of a nuclear reactor or particle accelerator.
Q: What is a General License?
A: A General License is a type of license that is granted to perform specific activities without the need for individual license application.
Q: Who issues the NRC Form 483?
A: The NRC (Nuclear Regulatory Commission) issues the NRC Form 483.
Q: Do I need an NRC Form 483 to perform in vitro testing with byproduct material?
A: Yes, you need an NRC Form 483 registration certificate to perform in vitro testing with byproduct material under a General License.
Form Details:
- Released on April 1, 2018;
- The latest available edition released by the U.S. Nuclear Regulatory Commission;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of NRC Form 483 by clicking the link below or browse more documents and templates provided by the U.S. Nuclear Regulatory Commission.