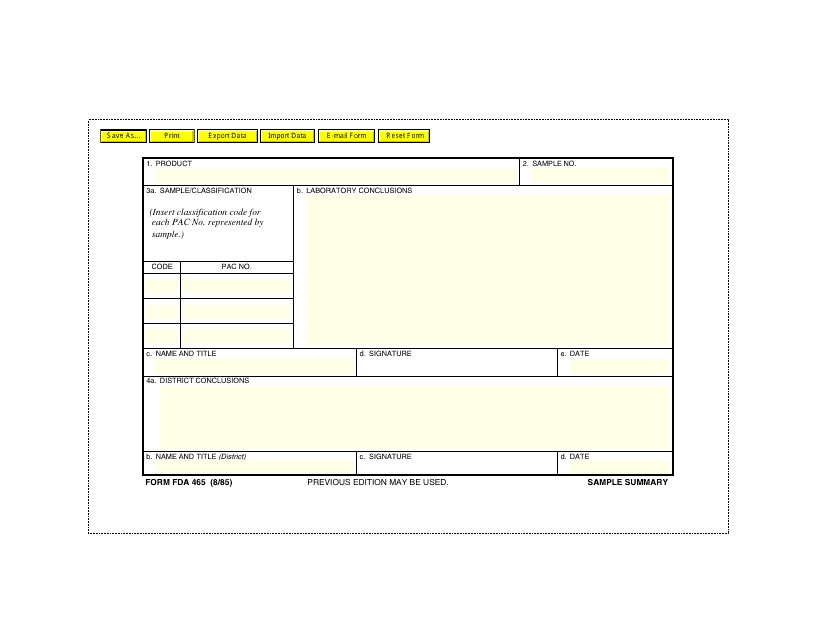
Form FDA465 Sample Summary
What Is Form FDA465?
This is a legal form that was released by the U.S. Department of Health and Human Services - U.S. Food and Drug Administration on August 1, 1985 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form FDA465?
A: Form FDA465 is a sample summary form used by the FDA.
Q: What is the purpose of Form FDA465?
A: The purpose of Form FDA465 is to summarize the information on samples collected by the FDA.
Q: Who uses Form FDA465?
A: Form FDA465 is used by the FDA.
Q: What information is included in Form FDA465?
A: Form FDA465 includes information about the collected samples, such as product identification, lot number, and laboratory analysis results.
Q: Why is Form FDA465 important?
A: Form FDA465 is important as it helps the FDA keep track of the collected samples and their analysis results.
Form Details:
- Released on August 1, 1985;
- The latest available edition released by the U.S. Department of Health and Human Services - U.S. Food and Drug Administration;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form FDA465 by clicking the link below or browse more documents and templates provided by the U.S. Department of Health and Human Services - U.S. Food and Drug Administration.