Form FDA421 Sample Accountability Record
What Is Form FDA421?
This is a legal form that was released by the U.S. Department of Health and Human Services - U.S. Food and Drug Administration on July 1, 2003 and used country-wide. As of today, no separate filing guidelines for the form are provided by the issuing department.
FAQ
Q: What is Form FDA421?
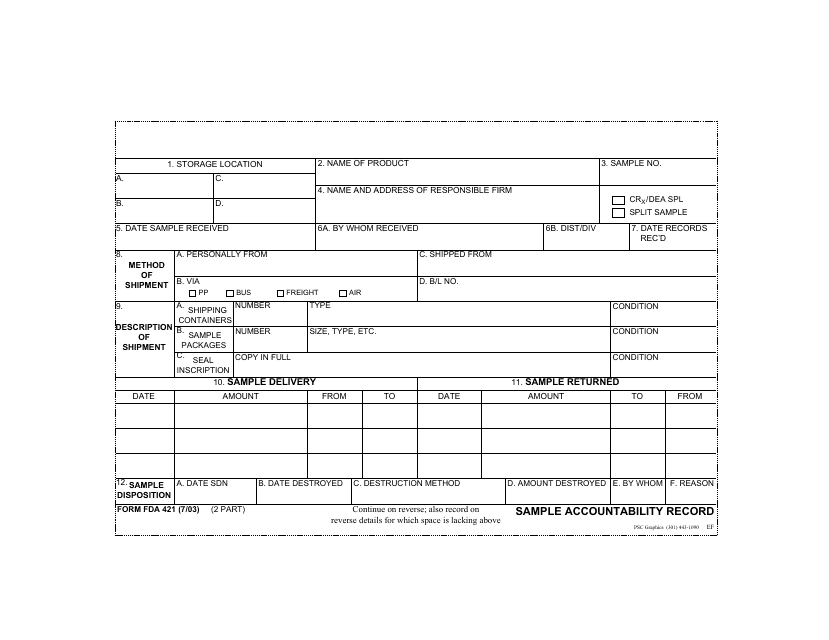
A: Form FDA421 is a Sample Accountability Record used by the U.S. Food and Drug Administration (FDA).
Q: What is the purpose of Form FDA421?
A: The purpose of Form FDA421 is to track and document the distribution and handling of samples by FDA investigators and compliance officers.
Q: Who uses Form FDA421?
A: Form FDA421 is used by FDA investigators and compliance officers who are responsible for handling and distributing samples in their investigations and inspections.
Q: What information is recorded on Form FDA421?
A: Form FDA421 records information such as the sample date, sample description, recipient information, and any follow-up actions taken.
Q: Is Form FDA421 mandatory?
A: Yes, Form FDA421 is a mandatory form that must be completed by FDA investigators and compliance officers when handling and distributing samples.
Form Details:
- Released on July 1, 2003;
- The latest available edition released by the U.S. Department of Health and Human Services - U.S. Food and Drug Administration;
- Easy to use and ready to print;
- Yours to fill out and keep for your records;
- Compatible with most PDF-viewing applications;
- Fill out the form in our online filing application.
Download a fillable version of Form FDA421 by clicking the link below or browse more documents and templates provided by the U.S. Department of Health and Human Services - U.S. Food and Drug Administration.