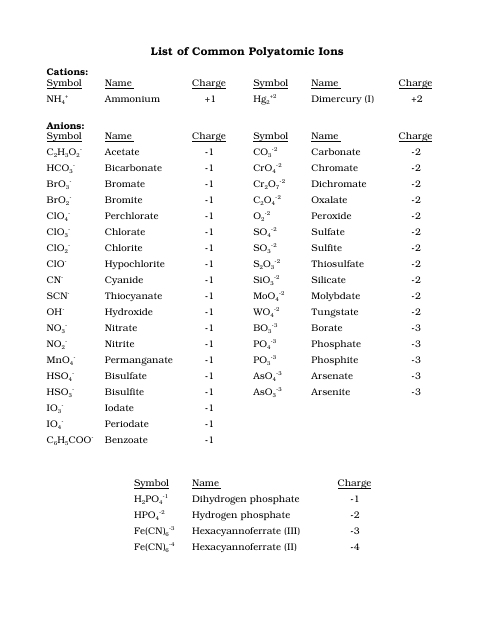
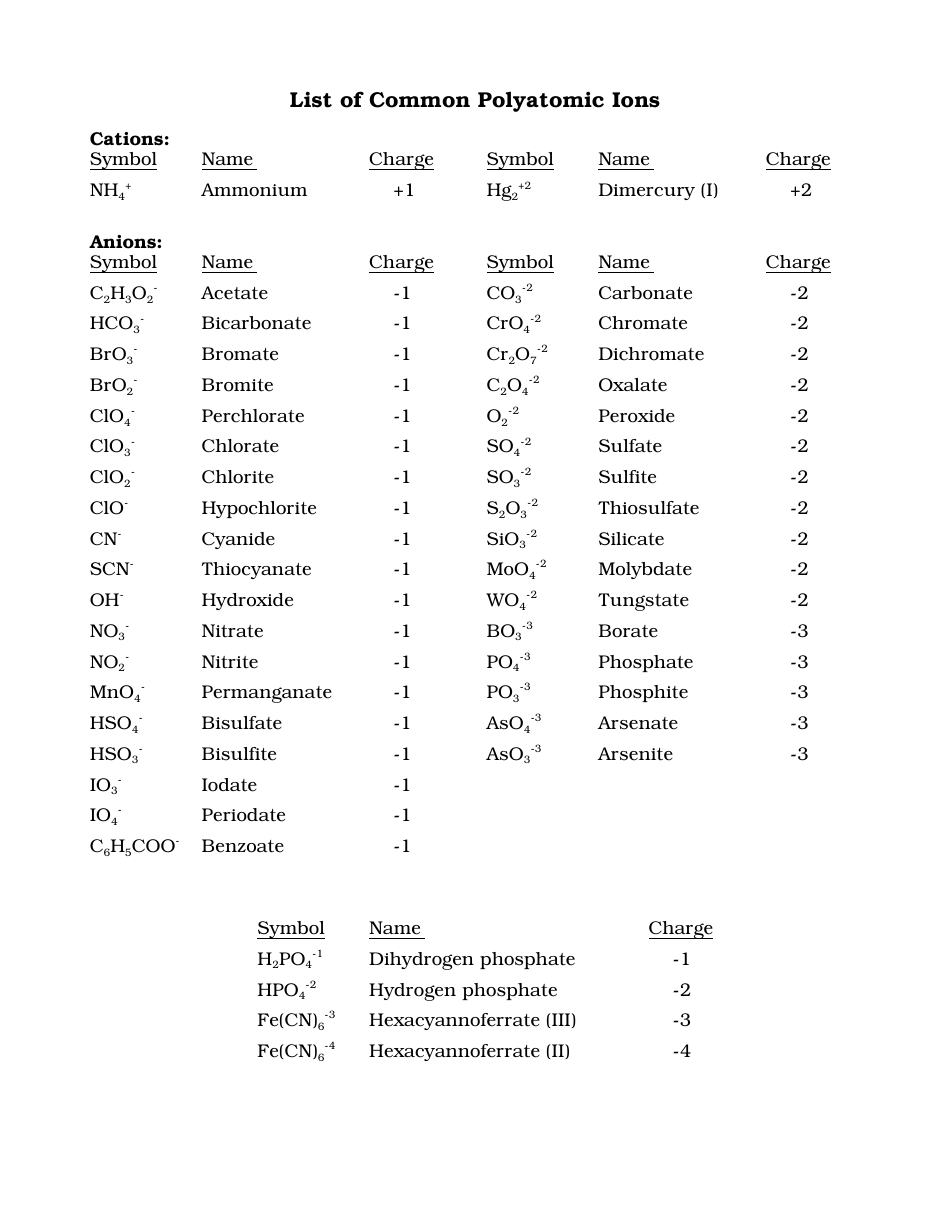
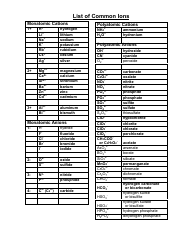
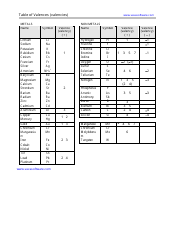
Common Polyatomic Ions Chart - Cations, Anions
The Common Polyatomic Ions Chart - Cations, Anions is used to identify and understand the different types of ions that form when atoms combine. It helps in studying chemical reactions and balancing equations.
The common polyatomic ions chart is typically not filed by a specific individual or organization. It is a resource that is widely available and can be found in various chemistry textbooks, online educational websites, and reference materials.
FAQ
Q: What is a polyatomic ion?
A: A polyatomic ion is a charged ion composed of two or more atoms.
Q: What is a cation?
A: A cation is a positively charged ion.
Q: What is an anion?
A: An anion is a negatively charged ion.
Q: What are some examples of common cations?
A: Some examples of common cations are sodium (Na+), potassium (K+), and calcium (Ca2+).
Q: What are some examples of common anions?
A: Some examples of common anions are chloride (Cl-), nitrate (NO3-), and sulfate (SO4^2-).
Q: What is the charge of the ammonium ion?
A: The ammonium ion (NH4+) has a charge of +1.
Q: What is the charge of the carbonate ion?
A: The carbonate ion (CO3^2-) has a charge of -2.
Q: What is the charge of the hydroxide ion?
A: The hydroxide ion (OH-) has a charge of -1.
Q: What is the charge of the phosphate ion?
A: The phosphate ion (PO4^3-) has a charge of -3.
Q: What is the charge of the sulfate ion?
A: The sulfate ion (SO4^2-) has a charge of -2.