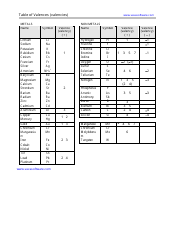
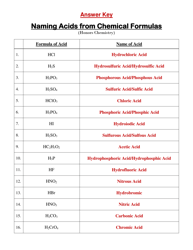
Chem 20 Review Sheet - Unit: Aqueous Solutions, Lesson: #1
The Chem 20 Review Sheet - Unit: Aqueous Solutions, Lesson: #1 is a study guide or summary document to prepare for a lesson on Aqueous Solutions in the chemistry course Chem 20. It may include key concepts, formulas, and practice problems related to the topic.
FAQ
Q: What are aqueous solutions?
A: Aqueous solutions are solutions where the solvent is water.
Q: Why is water a good solvent?
A: Water is a good solvent because it has a high polarity and can dissolve many substances.
Q: What does it mean for a substance to be soluble?
A: A substance is soluble if it can dissolve in a given solvent.
Q: What are the three types of aqueous solutions?
A: The three types of aqueous solutions are electrolyte solutions, non-electrolyte solutions, and solute gases dissolved in water.
Q: What is an electrolyte?
A: An electrolyte is a substance that dissociates into ions when dissolved in water, allowing it to conduct electricity.