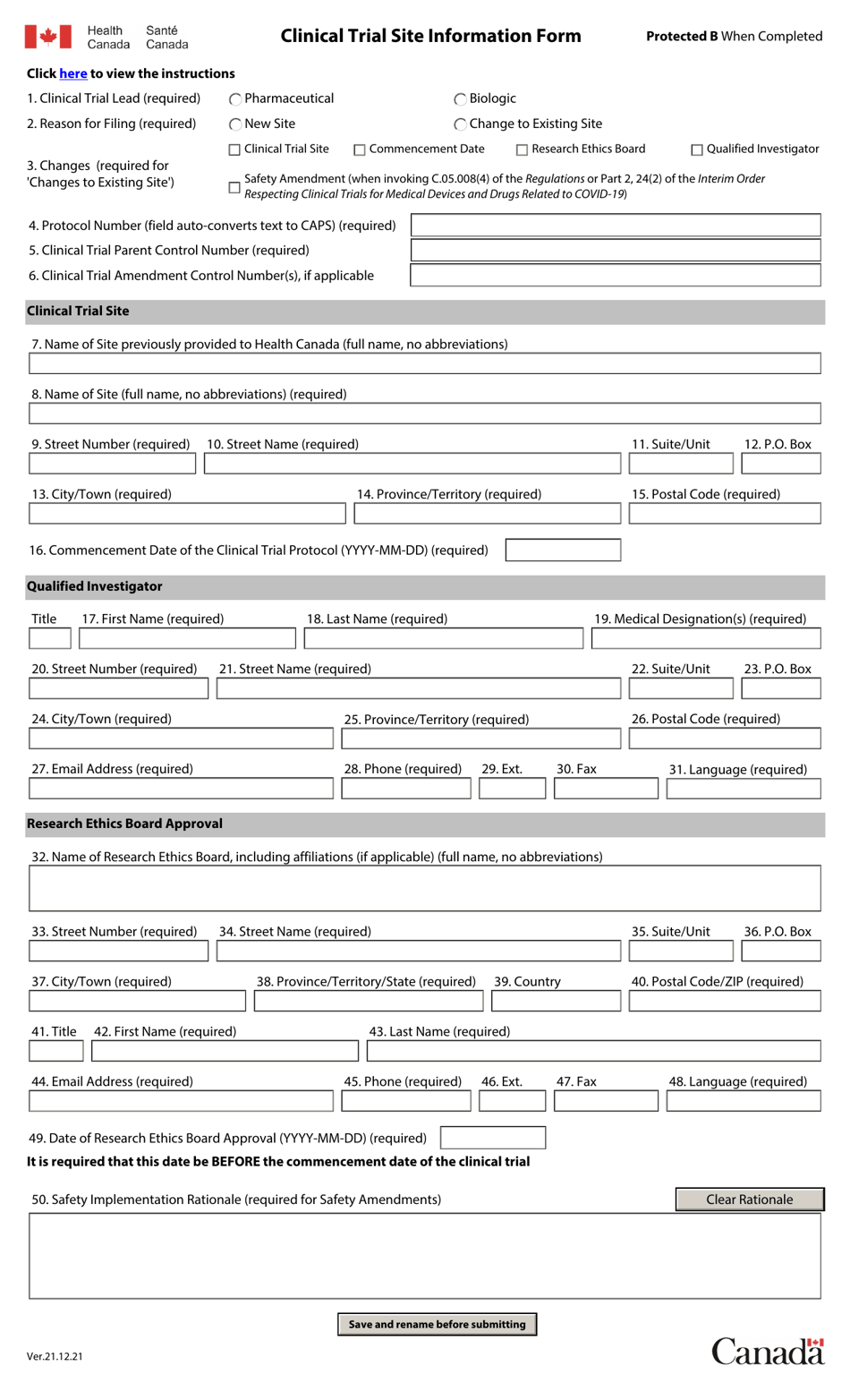
Clinical Trial Site Information Form - Canada
The Clinical Trial Site Information Form - Canada is used to collect information about the sites where clinical trials are conducted in Canada. This form helps to ensure transparency, accuracy, and consistency in clinical trial data collection and reporting processes.
The clinical trial site in Canada typically files the Clinical Trial Site Information Form.
Clinical Trial Site Information Form - Canada - Frequently Asked Questions (FAQ)
Q: What is a Clinical Trial Site Information Form?
A: The Clinical Trial Site Information Form is a document that collects information about a specific location where a clinical trial will take place in Canada.
Q: Why is the Clinical Trial Site Information Form necessary?
A: The form is necessary to ensure that the clinical trial site meets the requirements and regulations set by the health authorities in Canada.
Q: What information is collected in the Clinical Trial Site Information Form?
A: The form collects information such as the name and address of the site, details about the principal investigator and research team, the study protocol, and any previous experience the site has with clinical trials.
Q: Who is responsible for filling out the Clinical Trial Site Information Form?
A: The principal investigator or the research team at the trial site are responsible for filling out the form.
Q: Are there any fees associated with submitting the Clinical Trial Site Information Form?
A: Fees may be required for the review and processing of the form by the Canadian regulatory authorities.
Q: What happens after the Clinical Trial Site Information Form is submitted?
A: After the form is submitted, it will be reviewed by the Canadian regulatory authorities to ensure compliance with the regulations. If approved, the site can proceed with the clinical trial.
Q: Can a site participate in a clinical trial without completing the Clinical Trial Site Information Form?
A: No, the completion of the Clinical Trial Site Information Form is a requirement for a site to participate in a clinical trial in Canada.