Form HC3011 Drug Submission Application Form for: Human, Veterinary or Disinfectant Drugs and Clinical Trial Application / Attestation - Canada
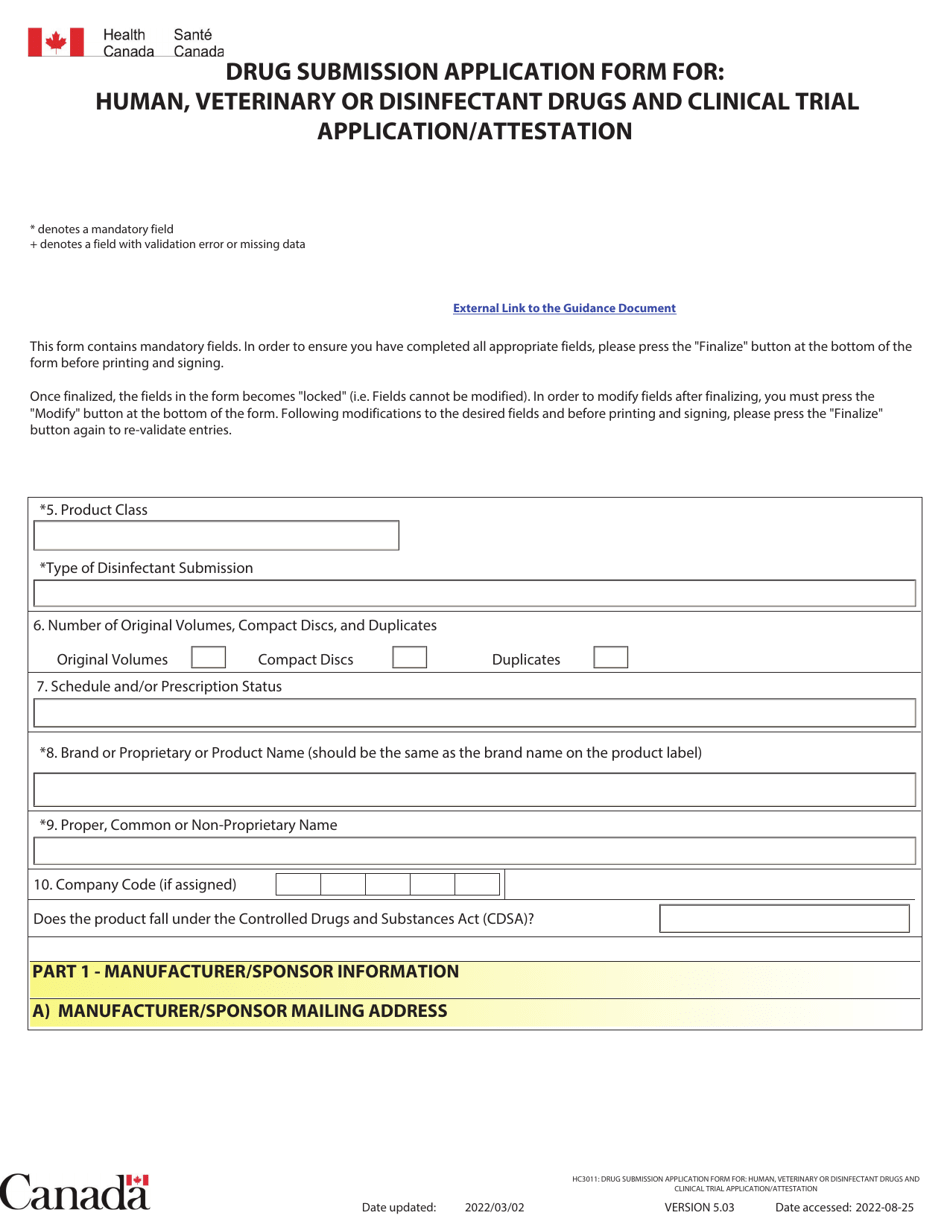
Form HC3011 Drug Submission Application Form is used in Canada for applying to get authorization to sell, import, or distribute human, veterinary, or disinfectant drugs. It is also used for the submission of clinical trial applications.
The applicant who wants to submit a drug submission application form for human, veterinary, or disinfectant drugs and clinical trial application/attestation in Canada files the Form HC3011.
Form HC3011 Drug Submission Application Form for: Human, Veterinary or Disinfectant Drugs and Clinical Trial Application/Attestation - Canada - Frequently Asked Questions (FAQ)
Q: What is the HC3011 Drug Submission Application Form used for?
A: The HC3011 Drug Submission Application Form is used for submitting applications for human, veterinary, disinfectant drugs, and clinical trials in Canada.
Q: Who can use the HC3011 Drug Submission Application Form?
A: The form can be used by individuals or companies seeking to obtain approval for human, veterinary, or disinfectant drugs, as well as those conducting clinical trials in Canada.
Q: What information is required in the HC3011 Drug Submission Application Form?
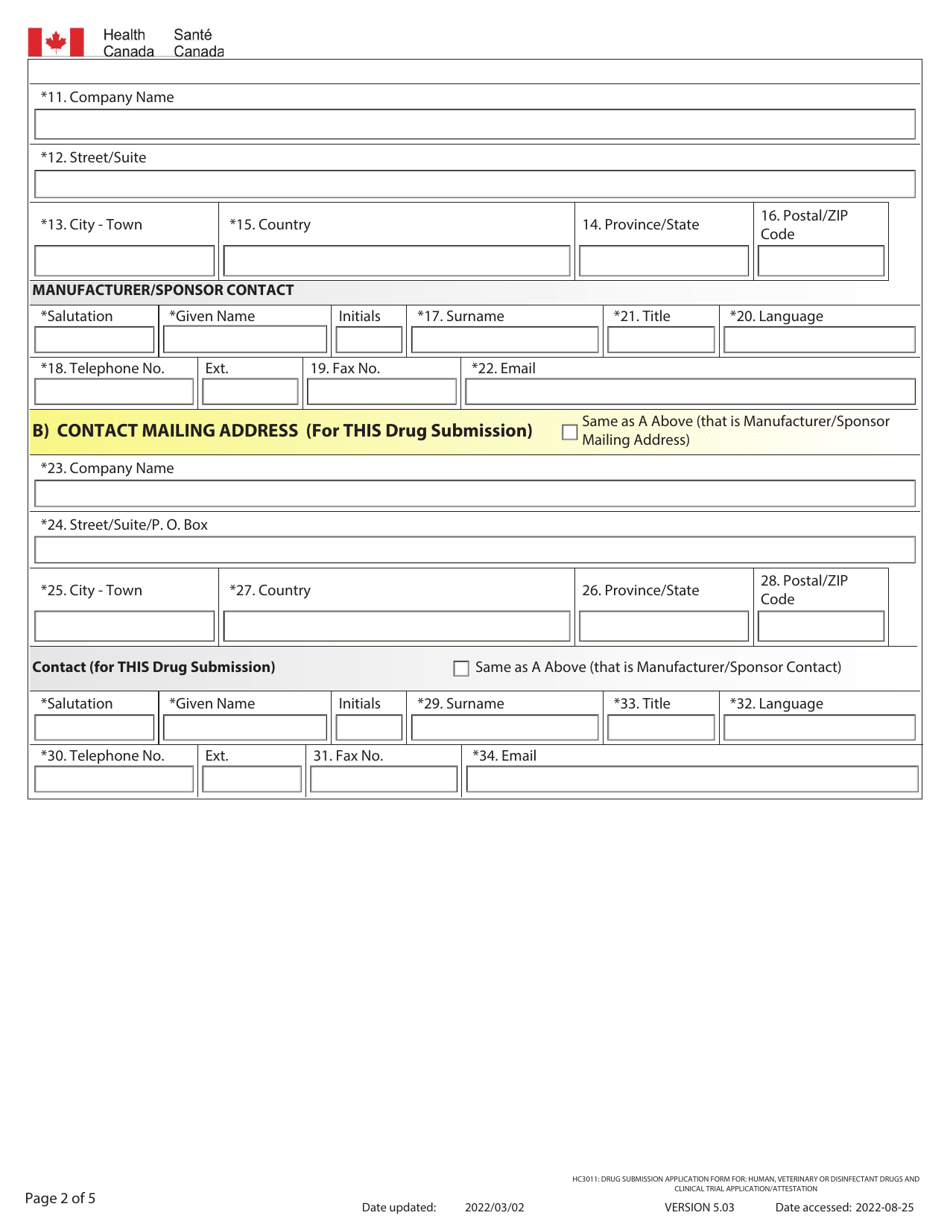
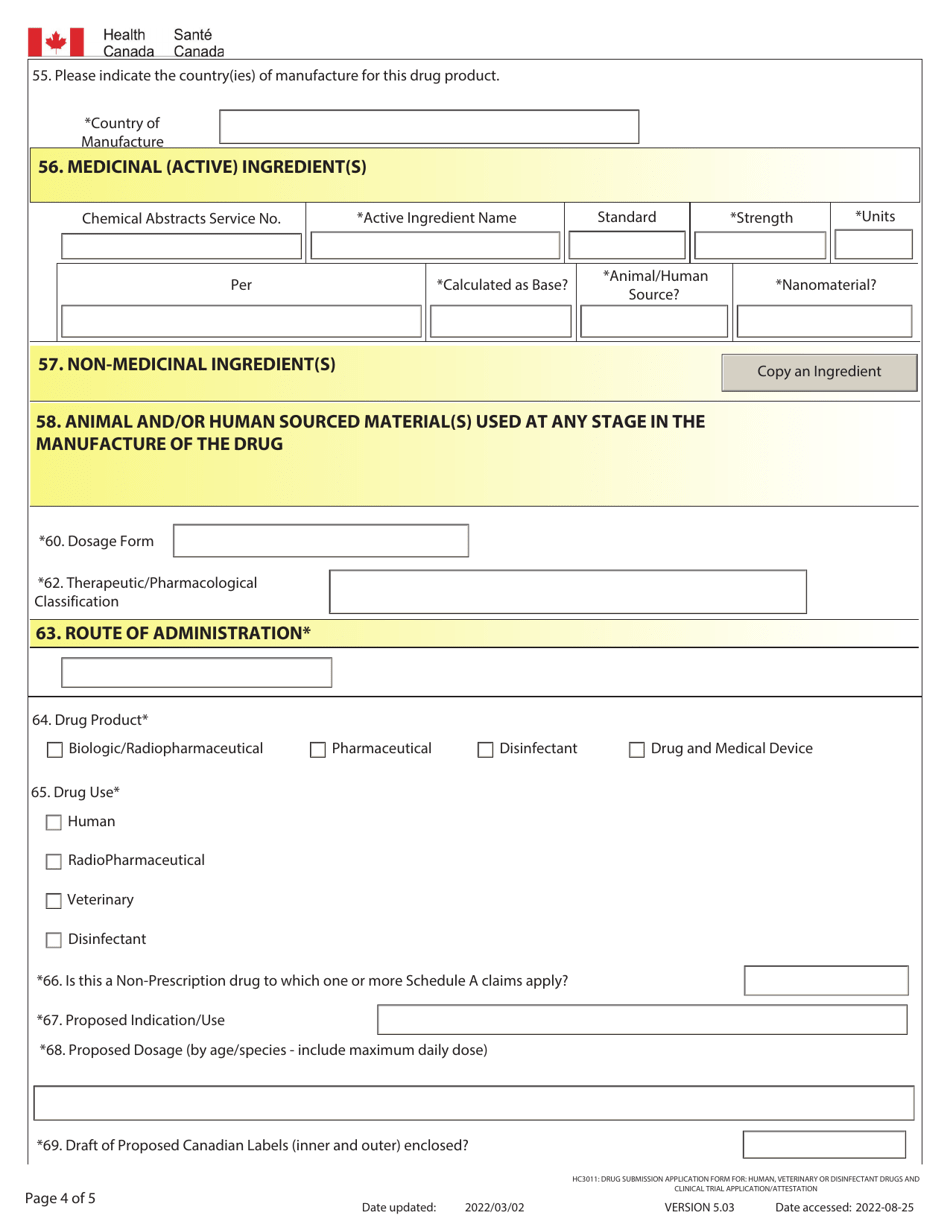
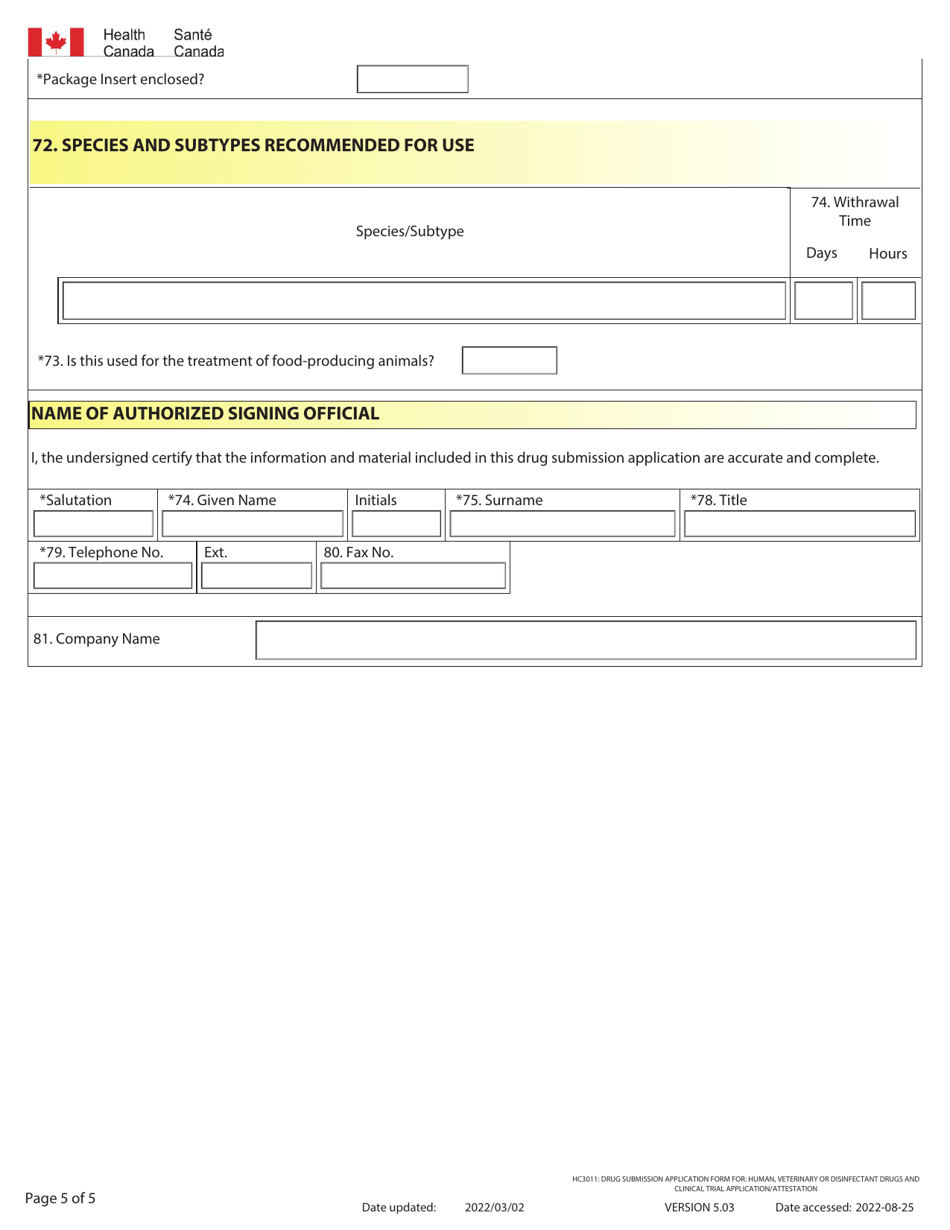
A: The form requires detailed information about the drug or trial, including its purpose, composition, manufacturing/packaging details, proposed labeling, safety and efficacy data, and more.