Research Compliance Templates
Are you conducting research or involved in any scientific studies? Ensuring that you adhere to research compliance guidelines is of utmost importance. Our comprehensive collection of documents, also known as research compliance or institutional assurance, is designed to assist you in navigating the complex world of research regulations.
Our extensive library includes a diverse range of forms, reports, and certifications that cover various aspects of research compliance. Whether you need to submit a Counting Study Reporting Form, a VA Form 10-0398 Research Protocol Safety Survey, a Form PHS-6349 Institutional Assurance and Annual Report on Possible Research Misconduct, an APHIS Form 7023 Annual Report of Research Facility, or a Research Ethics Board Attestation (also applicable in Canada), we have you covered.
Our research compliance documents are meticulously drafted to meet the standards set by regulatory authorities. These resources provide researchers, institutions, and organizations with the necessary tools to ensure ethical conduct, participant safety, and overall compliance.
Stay on the right track with our research compliance collection, and rest assured that your research endeavors are carried out in accordance with the established guidelines and protocols. Trust in our comprehensive library to streamline your research compliance endeavors and mitigate potential risks.
Documents:
7
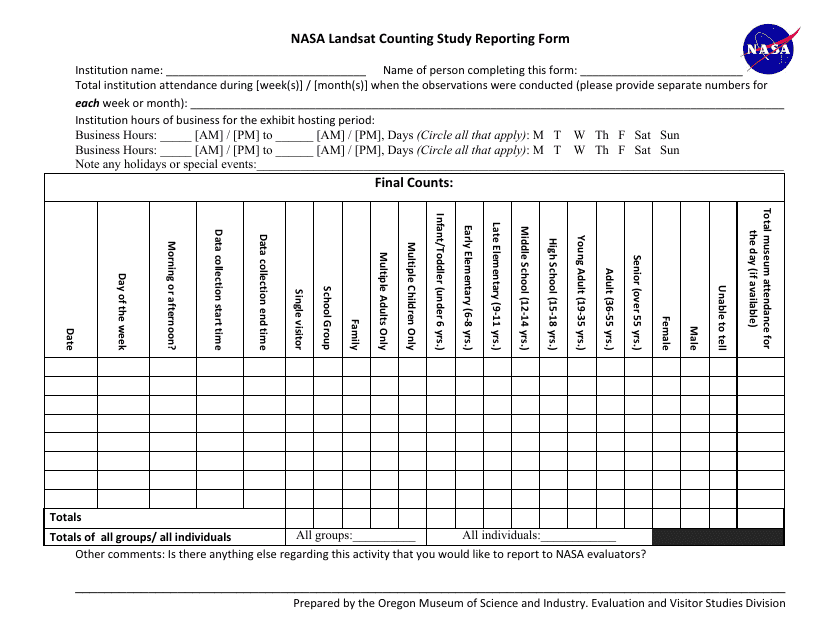
This Form is used for reporting the results of a counting study. It is used to document and summarize the data collected during a counting study, which is a research method used to quantify or assess the number of items or occurrences of interest.
This document is used for the mandatory routing of sponsored research at Centre College. It ensures proper approval and administration of funded research projects.
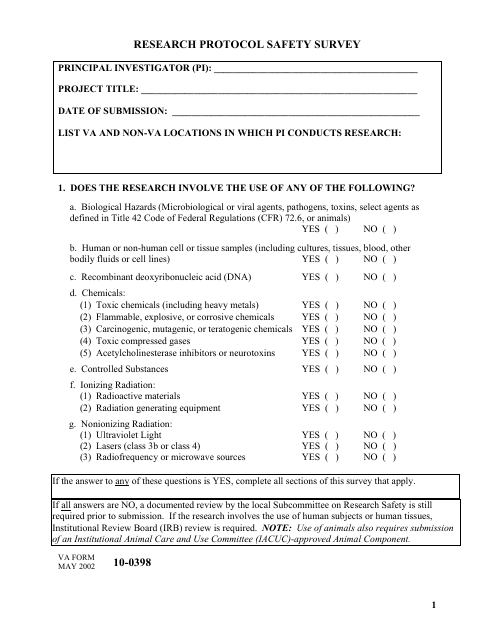
This form is used for conducting a safety survey for research protocols.
This Form is used for individuals or organizations who want to modify their existing human subjects research in the state of New Jersey. It provides instructions on how to complete the IRB-3 application.
This document is for attesting to the ethics approval from the Research Ethics Board in Canada. It is required for conducting research studies in compliance with ethical guidelines.