Drug Regulation Templates
Welcome to our webpage on drug regulation. As an important aspect of public health and safety, drug regulations play a crucial role in ensuring the quality, safety, and efficacy of pharmaceutical products. Our comprehensive collection of documents on drug regulation provides valuable information and resources for healthcare professionals, regulatory agencies, and the general public.
From information about controlled substances registers to fact sheets from the Drug Enforcement Administration (DEA), our collection covers a wide range of topics related to drug regulation. Whether you are a pharmacist, a healthcare provider, or a regulatory authority, you will find relevant and up-to-date information to support your work in this field.
Our collection includes documents such as the Pharmacist in Charge (PIC) Change Form, which allows pharmacists in South Dakota to update their information with the relevant authorities. Additionally, we offer resources like the Frequent Dispensing Authorization form for healthcare professionals in British Columbia, Canada, allowing them to provide the appropriate level of care to their patients.
For policymakers and healthcare administrators, our State Drug Policy Fill-In forms provide the necessary template to develop and implement effective drug policies. These forms can help shape regulations and guidelines that contribute to the safe and responsible use of medications.
At our webpage, you'll find a wealth of resources and information on drug regulation, helping you navigate the complex landscape of pharmaceutical oversight. Stay informed, stay compliant, and stay committed to ensuring the highest standards in drug regulation. Explore our collection of documents today to learn more.
Documents:
8
This form is used for attesting to the details of a non-prescription drug monograph in Canada.
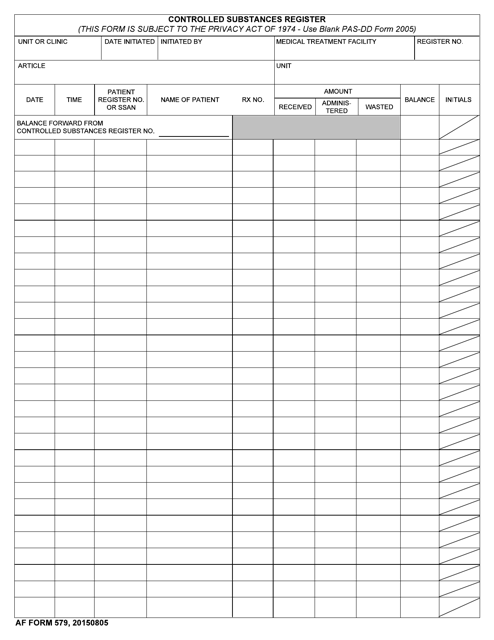
This form is used for keeping track of controlled substances in a secure and controlled manner. It helps maintain accurate records of the quantities and usage of controlled substances.
This form is used for reporting serious adverse drug reactions that occur in hospitals in Canada.
This document provides information about the Drug Enforcement Administration (DEA), its mission, and its responsibilities in combating drug trafficking and abuse in the United States. It also highlights key statistics and facts related to the DEA's work.
This document is used for requesting a change of the Pharmacist in Charge (PIC) in South Dakota.
This document is a fill-in form for the State Drug Policy in Arkansas. It contains information and fields that need to be filled in regarding the state's policies and regulations on drugs.